PAK3 Exacerbates Cardiac Lipotoxicity via SREBP1c in Obesity Cardiomyopathy
- PMID: 39137120
- PMCID: PMC11493761
- DOI: 10.2337/db24-0240
PAK3 Exacerbates Cardiac Lipotoxicity via SREBP1c in Obesity Cardiomyopathy
Abstract
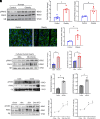
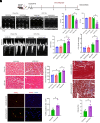
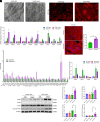
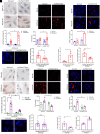
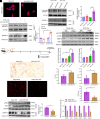
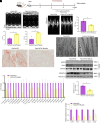
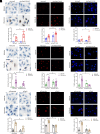
Obesity-induced lipid overload in cardiomyocytes contributes to profound oxidative stress and cardiomyopathy, culminating in heart failure. In this study, we investigate a novel mechanism whereby lipids accumulate in cardiomyocytes, and seek the relevant treatment strategies. P21-activated kinase 3 (PAK3) was elevated in obese human myocardium, and the murine hearts and cardiomyocytes upon diet- or fatty acid-induced stress, respectively. Mice with cardiac-specific overexpression of PAK3 were more susceptible to the development of cardiac dysfunction upon diet stress, at least partially, because of increased deposition of toxic lipids within the myocardium. Mechanistically, PAK3 promoted the nuclear expression of sterol regulatory element binding protein 1c (SREBP1c) through activation of mammalian target of rapamycin (mTOR) and ribosomal protein S6 kinase β-1 (S6K1) pathway in cardiomyocytes, resulting in abnormal lipid genes profile, accumulation of excessive lipids, and oxidative stress. More importantly, PAK3 knockdown attenuated fatty acid-induced lipotoxicity and cell death in rat and human cardiomyocytes. More importantly, the S6K1 or SREBP1c inhibitor alleviated PAK3-triggered intracellular lipid overload and cardiac dysfunction under obese stress. Collectively, we have demonstrated that PAK3 impairs myocardial lipid homeostasis, while inhibition of cardiac lipotoxicity mitigates cardiac dysfunction. Our study provides a promising therapeutic strategy for ameliorating obesity cardiomyopathy.
© 2024 by the American Diabetes Association.
Conflict of interest statement
Figures

References
-
- Powell-Wiley TM, Poirier P, Burke LE, et al.; American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council . Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2021;143:e984–e1010 - PMC - PubMed
-
- Tong M, Saito T, Zhai P, et al. Alternative mitophagy protects the heart against obesity-associated cardiomyopathy. Circ Res 2021;129:1105–1121 - PubMed
-
- Nakamura M, Sadoshima J. Cardiomyopathy in obesity, insulin resistance and diabetes. J Physiol 2020;598:2977–2993 - PubMed
-
- Ouwens DM, Boer C, Fodor M, et al. Cardiac dysfunction induced by high-fat diet is associated with altered myocardial insulin signalling in rats. Diabetologia 2005;48:1229–1237 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

