Identification of factors directly linked to incident chronic obstructive pulmonary disease: A causal graph modeling study
- PMID: 39137208
- PMCID: PMC11349214
- DOI: 10.1371/journal.pmed.1004444
Identification of factors directly linked to incident chronic obstructive pulmonary disease: A causal graph modeling study
Abstract
Background: Beyond exposure to cigarette smoking and aging, the factors that influence lung function decline to incident chronic obstructive pulmonary disease (COPD) remain unclear. Advancements have been made in categorizing COPD into emphysema and airway predominant disease subtypes; however, predicting which healthy individuals will progress to COPD is difficult because they can exhibit profoundly different disease trajectories despite similar initial risk factors. This study aimed to identify clinical, genetic, and radiological features that are directly linked-and subsequently predict-abnormal lung function.
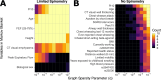
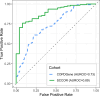
Methods and findings: We employed graph modeling on 2,643 COPDGene participants (aged 45 to 80 years, 51.25% female, 35.1% African Americans; enrollment 11/2007-4/2011) with smoking history but normal spirometry at study enrollment to identify variables that are directly linked to future lung function abnormalities. We developed logistic regression and random forest predictive models for distinguishing individuals who maintain lung function from those who decline. Of the 131 variables analyzed, 6 were identified as informative to future lung function abnormalities, namely forced expiratory flow in the middle range (FEF25-75%), average lung wall thickness in a 10 mm radius (Pi10), severe emphysema, age, sex, and height. We investigated whether these features predict individuals leaving GOLD 0 status (normal spirometry according to Global Initiative for Obstructive Lung Disease (GOLD) criteria). Linear models, trained with these features, were quite predictive (area under receiver operator characteristic curve or AUROC = 0.75). Random forest predictors performed similarly to logistic regression (AUROC = 0.7), indicating that no significant nonlinear effects were present. The results were externally validated on 150 participants from Specialized Center for Clinically Oriented Research (SCCOR) cohort (aged 45 to 80 years, 52.7% female, 4.7% African Americans; enrollment: 7/2007-12/2012) (AUROC = 0.89). The main limitation of longitudinal studies with 5- and 10-year follow-up is the introduction of mortality bias that disproportionately affects the more severe cases. However, our study focused on spirometrically normal individuals, who have a lower mortality rate. Another limitation is the use of strict criteria to define spirometrically normal individuals, which was unavoidable when studying factors associated with changes in normalized forced expiratory volume in 1 s (FEV1%predicted) or the ratio of FEV1/FVC (forced vital capacity).
Conclusions: This study took an agnostic approach to identify which baseline measurements differentiate and predict the early stages of lung function decline in individuals with previous smoking history. Our analysis suggests that emphysema affects obstruction onset, while airway predominant pathology may play a more important role in future FEV1 (%predicted) decline without obstruction, and FEF25-75% may affect both.
Copyright: © 2024 Gregg et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
RWG, CMK, and PVB have no competing interests. FCS has received grant support and consulting fees from Sanofi/Regeneron, AstraZeneca, Verona Pharma, Nuvaira, Gala Therapeutics, GlaxoSmithKline, Boehringer Ingelheim. EKS has received grant support from Bayer and Northpond Laboratories. DLD has received grant support from Bayer and the Alpha-1 Foundation.
Figures



References
-
- Chronic obstructive pulmonary disease (COPD). Available from: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pul.... Accessed 2023 Jan 6.
-
- Pauwels RA, Buist AS, Calverley PMA, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039 - DOI - PubMed
MeSH terms
Grants and funding
- 75N93023D00011/AI/NIAID NIH HHS/United States
- U01 HL089897/HL/NHLBI NIH HHS/United States
- U01 HL089856/HL/NHLBI NIH HHS/United States
- R01 HG011393/HG/NHGRI NIH HHS/United States
- R01 HL157879/HL/NHLBI NIH HHS/United States
- T15 LM007059/LM/NLM NIH HHS/United States
- 75N90023D00011/CL/CLC NIH HHS/United States
- R01 HL159805/HL/NHLBI NIH HHS/United States
- P01 HL132825/HL/NHLBI NIH HHS/United States
- P01 HL114501/HL/NHLBI NIH HHS/United States
- R01 HL152735/HL/NHLBI NIH HHS/United States
- 75N92021D00011/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

