YAP1 Inhibition Induces Phenotype Switching of Cancer-Associated Fibroblasts to Tumor Suppressive in Prostate Cancer
- PMID: 39137404
- PMCID: PMC11565174
- DOI: 10.1158/0008-5472.CAN-24-0932
YAP1 Inhibition Induces Phenotype Switching of Cancer-Associated Fibroblasts to Tumor Suppressive in Prostate Cancer
Abstract
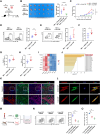
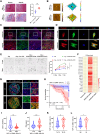
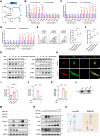
Prostate cancer rarely responds to immune-checkpoint blockade (ICB) therapies. Cancer-associated fibroblasts (CAF) are critical components of the immunologically "cold" tumor microenvironment and are considered a promising target to enhance the immunotherapy response. In this study, we aimed to reveal the mechanisms regulating CAF plasticity to identify potential strategies to switch CAFs from protumorigenic to antitumor phenotypes and to enhance ICB efficacy in prostate cancer. Integration of four prostate cancer single-cell RNA sequencing datasets defined protumorigenic and antitumor CAFs, and RNA-seq, flow cytometry, and a prostate cancer organoid model demonstrated the functions of two CAF subtypes. Extracellular matrix-associated CAFs (ECM-CAF) promoted collagen deposition and cancer cell progression, and lymphocyte-associated CAFs (Lym-CAF) exhibited an antitumor phenotype and induced the infiltration and activation of CD8+ T cells. YAP1 activity regulated the ECM-CAF phenotype, and YAP1 silencing promoted switching to Lym-CAFs. NF-κB p65 was the core transcription factor in the Lym-CAF subset, and YAP1 inhibited nuclear translocation of p65. Selective depletion of YAP1 in ECM-CAFs in vivo promoted CD8+ T-cell infiltration and activation and enhanced the therapeutic effects of anti-PD-1 treatment on prostate cancer. Overall, this study revealed a mechanism regulating CAF identity in prostate cancer and highlighted a therapeutic strategy for altering the CAF subtype to suppress tumor growth and increase sensitivity to ICB. Significance: YAP1 regulates cancer-associated fibroblast phenotypes and can be targeted to switch cancer-associated fibroblasts from a protumorigenic subtype that promotes extracellular matrix deposition to a tumor-suppressive subtype that stimulates antitumor immunity and immunotherapy efficacy.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
No disclosures were reported.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

