Addressing the knowledge gap in the genomic landscape and tailored therapeutic approaches to adolescent and young adult cancers
- PMID: 39137480
- PMCID: PMC11369407
- DOI: 10.1016/j.esmoop.2024.103659
Addressing the knowledge gap in the genomic landscape and tailored therapeutic approaches to adolescent and young adult cancers
Abstract
Background: Adolescents and young adults (AYAs) represent a small proportion of patients with cancer. The genomic profiles of AYA patients with cancer are not well-studied, and outcomes of genome-matched therapies remain largely unknown.
Patients and methods: We investigated differences between Japanese AYA and older adult (OA) patients in genomic alterations, therapeutic evidence levels, and genome-matched therapy usage by cancer type. We also assessed treatment outcomes.
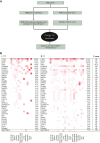
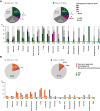
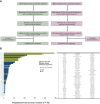
Results: AYA patients accounted for 8.3% of 876 cases. Microsatellite instability-high and/or tumor mutation burden was less common in AYA patients (1.4% versus 7.7% in OA; P = 0.05). However, BRCA1 alterations were more common in AYA patients with breast cancer (27.3% versus 1.7% in OA; P = 0.01), as were MYC alterations in AYA patients with colorectal cancer (23.5% versus 5.8% in OA; P = 0.02) and sarcoma (31.3% versus 3.4% in OA; P = 0.01). Genome-matched therapy use was similar between groups, with overall survival tending to improve in both. However, in AYA patients, the small number of patients prevented statistical significance. Comprehensive genomic profiling-guided genome-matched therapy yielded encouraging results, with progression-free survival of 9.0 months in AYA versus 3.7 months in OA patients (P = 0.59).
Conclusion: Our study suggests that tailored therapeutic approaches can benefit cancer patients regardless of age.
Keywords: adolescent; genome-matched therapy; genomic profiling; older adult; treatment outcome; young adult.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- National Cancer Institute The Surveillance, Epidemiology, and End Results (SEER) Program. https://seer.cancer.gov/ Available at.
-
- Fern L.A., Lewandowski J.A., Coxon K.M., et al. Available, accessible, aware, appropriate, and acceptable: a strategy to improve participation of teenagers and young adults in cancer trials. Lancet Oncol. 2014;15(8):e341–e350. - PubMed
-
- Bhatia S., Pappo A.S., Acquazzino M., et al. Adolescent and young adult (AYA) oncology, version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21(8):851–880. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

