The mitochondrial pyruvate carrier regulates adipose glucose partitioning in female mice
- PMID: 39137831
- PMCID: PMC11382204
- DOI: 10.1016/j.molmet.2024.102005
The mitochondrial pyruvate carrier regulates adipose glucose partitioning in female mice
Abstract
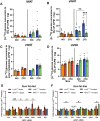
Objective: The mitochondrial pyruvate carrier (MPC) occupies a critical node in intermediary metabolism, prompting interest in its utility as a therapeutic target for the treatment of obesity and cardiometabolic disease. Dysregulated nutrient metabolism in adipose tissue is a prominent feature of obesity pathophysiology, yet the functional role of adipose MPC has not been explored. We investigated whether the MPC shapes the adaptation of adipose tissue to dietary stress in female and male mice.
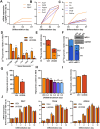
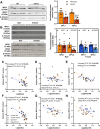
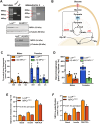
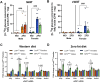
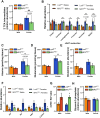
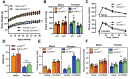
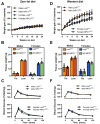
Methods: The impact of pharmacological and genetic disruption of the MPC on mitochondrial pathways of triglyceride assembly (lipogenesis and glyceroneogenesis) was assessed in 3T3L1 adipocytes and murine adipose explants, combined with analyses of adipose MPC expression in metabolically compromised humans. Whole-body and adipose-specific glucose metabolism were subsequently investigated in male and female mice lacking adipocyte MPC1 (Mpc1AD-/-) and fed either standard chow, high-fat western style, or high-sucrose lipid restricted diets for 24 weeks, using a combination of radiolabeled tracers and GC/MS metabolomics.
Results: Treatment with UK5099 or siMPC1 impaired the synthesis of lipids and glycerol-3-phosphate from pyruvate and blunted triglyceride accumulation in 3T3L1 adipocytes, whilst MPC expression in human adipose tissue was negatively correlated with indices of whole-body and adipose tissue metabolic dysfunction. Mature adipose explants from Mpc1AD-/- mice were intrinsically incapable of incorporating pyruvate into triglycerides. In vivo, MPC deletion restricted the incorporation of circulating glucose into adipose triglycerides, but only in female mice fed a zero fat diet, and this associated with sex-specific reductions in tricarboxylic acid cycle pool sizes and compensatory transcriptional changes in lipogenic and glycerol metabolism pathways. However, whole-body adiposity and metabolic health were preserved in Mpc1AD-/- mice regardless of sex, even under conditions of zero dietary fat.
Conclusions: These findings highlight the greater capacity for mitochondrially driven triglyceride assembly in adipose from female versus male mice and expose a reliance upon MPC-gated metabolism for glucose partitioning in female adipose under conditions of dietary lipid restriction.
Keywords: Adipose; Glyceroneogenesis; Lipogenesis; Mitochondria; Sexual dimorphism.
Copyright © 2024 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Update of
-
Sex-dependent adipose glucose partitioning by the mitochondrial pyruvate carrier.bioRxiv [Preprint]. 2024 May 14:2024.05.11.593540. doi: 10.1101/2024.05.11.593540. bioRxiv. 2024. Update in: Mol Metab. 2024 Oct;88:102005. doi: 10.1016/j.molmet.2024.102005. PMID: 38798427 Free PMC article. Updated. Preprint.
References
-
- Virtanen K.A., Peltoniemi P., Marjamäki P., Asola M., Strindberg L., Parkkola R., et al. Human adipose tissue glucose uptake determined using [(18)F]-fluoro-deoxy-glucose ([(18)F]FDG) and PET in combination with microdialysis. Diabetologia. 2001;44(12):2171–2179. doi: 10.1007/s001250100026. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

