γ1 GABAA Receptors in Spinal Nociceptive Circuits
- PMID: 39137998
- PMCID: PMC11466064
- DOI: 10.1523/JNEUROSCI.0591-24.2024
γ1 GABAA Receptors in Spinal Nociceptive Circuits
Abstract
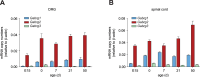
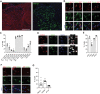
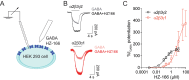
GABAergic neurons and GABAA receptors (GABAARs) are critical elements of almost all neuronal circuits. Most GABAARs of the CNS are heteropentameric ion channels composed of two α, two β, and one γ subunits. These receptors serve as important drug targets for benzodiazepine (BDZ) site agonists, which potentiate the action of GABA at GABAARs. Most GABAAR classifications rely on the heterogeneity of the α subunit (α1-α6) included in the receptor complex. Heterogeneity of the γ subunits (γ1-γ3), which mediate synaptic clustering of GABAARs and contribute, together with α subunits, to the benzodiazepine (BDZ) binding site, has gained less attention, mainly because γ2 subunits greatly outnumber the other γ subunits in most brain regions. Here, we have investigated a potential role of non-γ2 GABAARs in neural circuits of the spinal dorsal horn, a key site of nociceptive processing. Female and male mice were studied. We demonstrate that besides γ2 subunits, γ1 subunits are significantly expressed in the spinal dorsal horn, especially in its superficial layers. Unlike global γ2 subunit deletion, which is lethal, spinal cord-specific loss of γ2 subunits was well tolerated. GABAAR clustering in the superficial dorsal horn remained largely unaffected and antihyperalgesic actions of HZ-166, a nonsedative BDZ site agonist, were partially retained. Our results thus suggest that the superficial dorsal horn harbors functionally relevant amounts of γ1 subunits that support the synaptic clustering of GABAARs in this site. They further suggest that γ1 containing GABAARs contribute to the spinal control of nociceptive information flow.
Keywords: GABAA receptor subtype; dorsal horn; gephyrin; nociception; pain; receptor clustering.
Copyright © 2024 Neumann et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Atack JR (2009) GABAA receptor α2/α3 subtype-selective modulators as potential nonsedating anxiolytics. In: Behavioral neurobiology of anxiety and its treatment. Current topics in behavioral neurosciences (Stein MB, Steckler T, eds), vol 2, pp 331–360. Berlin, Heidelberg, Germany: Springer. 10.1007/7854_2009_30 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
