Update on the pathogenesis of atopic dermatitis
- PMID: 39138034
- PMCID: PMC11551276
- DOI: 10.1016/j.abd.2024.06.001
Update on the pathogenesis of atopic dermatitis
Abstract
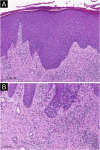
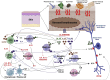
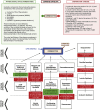
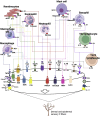
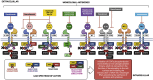
Atopic dermatitis is a chronic, recurrent, and multifactorial skin-mucosal manifestation resulting from the interaction between elements mainly associated with the skin barrier deficit, the homeostasis of the immune response, neurological aspects, and patterns of reactivity to environmental antigens, which are established in genetically predisposed individuals. In addition to the skin, atopic diathesis involves other organs such as the airways (upper and lower), eyes, digestive tract, and neuropsychiatric aspects, which inflict additional morbidity on the dermatological patient. The different phenotypes of the disease fundamentally depend on the participation of each of these factors, in different life circumstances, such as age groups, occupational exposure patterns, physical activity, pollution, genetic load, and climatic factors. A better understanding of the complexity of its pathogenesis allows not only the understanding of therapeutic targets but also how to identify preponderant elements that mediate disease activity in each circumstance, for selecting the best treatment strategies and mitigation of triggering factors. This narrative review presents an update on the pathogenesis of atopic dermatitis, especially aimed at understanding the clinical manifestations, the main disease phenotypes and the context of available therapeutic strategies.
Keywords: Atopic dermatitis; Cytokines; Dermatopathies; Eczema; Homeopathic pathogenesis; Immunity.
Copyright © 2024 Sociedade Brasileira de Dermatologia. Published by Elsevier España, S.L.U. All rights reserved.
Figures
References
-
- Drucker A.M., Wang A.R., Li W.Q., Sevetson E., Block J.K., Qureshi A.A. The burden of atopic dermatitis: summary of a report for the national eczema association. J Invest Dermatol. 2017;137:26–30. - PubMed
-
- Warschburger P., Buchholz H.T., Petermann F. Psychological adjustment in parents of young children with atopic dermatitis: which factors predict parental quality of life? Br J Dermatol. 2004;150:304–311. - PubMed
-
- de Re Lai M.R., Gaspar D.B., Proenca C.C., Mayor S.S., Miot H.A. Performance of the main clinical scores in the assessment of atopic dermatitis severity. Int J Dermatol. 2024;63:e8–e10. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

