Digital solutions in paediatric sepsis: current state, challenges, and opportunities to improve care around the world
- PMID: 39138095
- PMCID: PMC11371309
- DOI: 10.1016/S2589-7500(24)00141-9
Digital solutions in paediatric sepsis: current state, challenges, and opportunities to improve care around the world
Abstract
The digitisation of health care is offering the promise of transforming the management of paediatric sepsis, which is a major source of morbidity and mortality in children worldwide. Digital technology is already making an impact in paediatric sepsis, but is almost exclusively benefiting patients in high-resource health-care settings. However, digital tools can be highly scalable and cost-effective, and-with the right planning-have the potential to reduce global health disparities. Novel digital solutions, from wearable devices and mobile apps, to electronic health record-embedded decision support tools, have an unprecedented opportunity to transform paediatric sepsis research and care. In this Series paper, we describe the current state of digital solutions in paediatric sepsis around the world, the advances in digital technology that are enabling the development of novel applications, and the potential effect of advances in artificial intelligence in paediatric sepsis research and clinical care.
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license.
Conflict of interest statement
Declaration of interests RSW served on the data safety monitoring board of the GRACE trial (granulocyte-macrophage colony-stimulating factor for reversal of immunoparalysis in pediatric sepsis-induced multiple organ dysfunction syndrome), chairs the data safety monitoring board of the PRECISE study (personalized immunomodulation in pediatric sepsis-induced multiple organ dysfunction syndrome), and is a co-investigator on the SHIPSS trial (stress hydrocortisone for pediatric septic shock; R01HD096901). LNS-P has stock options in Celldom, Saccharo, Allyx Therapeutics, and InnoSIGN, which are companies focused on diagnostic and therapeutic approaches to cancer and Alzheimer's disease, not sepsis. HS has received support for travel to meetings related to sepsis quality improvement from the Children's Hospital Association, and travel support from the Society of Critical Care Medicine. RSW declares travel support from the Society of Critical Care Medicine, and is co-chair (unpaid) of the Pediatric Sepsis Definitions Task Force for the Society of Critical Care Medicine. LNS-P, HS, LJS, and TDB are members (unpaid) of the Pediatric Sepsis Definitions Task Force for the Society of Critical Care Medicine. All other authors declare no competing interests.
Figures
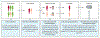


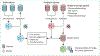
References
-
- Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med 2018; 6: 223–30. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

