The critical dynamics of hippocampal seizures
- PMID: 39138153
- PMCID: PMC11322644
- DOI: 10.1038/s41467-024-50504-9
The critical dynamics of hippocampal seizures
Abstract
Epilepsy is defined by the abrupt emergence of harmful seizures, but the nature of these regime shifts remains enigmatic. From the perspective of dynamical systems theory, such critical transitions occur upon inconspicuous perturbations in highly interconnected systems and can be modeled as mathematical bifurcations between alternative regimes. The predictability of critical transitions represents a major challenge, but the theory predicts the appearance of subtle dynamical signatures on the verge of instability. Whether such dynamical signatures can be measured before impending seizures remains uncertain. Here, we verified that predictions on bifurcations applied to the onset of hippocampal seizures, providing concordant results from in silico modeling, optogenetics experiments in male mice and intracranial EEG recordings in human patients with epilepsy. Leveraging pharmacological control over neural excitability, we showed that the boundary between physiological excitability and seizures can be inferred from dynamical signatures passively recorded or actively probed in hippocampal circuits. Of importance for the design of future neurotechnologies, active probing surpassed passive recording to decode underlying levels of neural excitability, notably when assessed from a network of propagating neural responses. Our findings provide a promising approach for predicting and preventing seizures, based on a sound understanding of their dynamics.
© 2024. The Author(s).
Conflict of interest statement
M.O.B. holds shares with Epios, Ltd., a medical device company based in Geneva. All other authors report no conflict of interest.
Figures



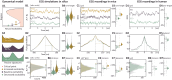



References
-
- Milton, J. G. & Jung, P. Epilepsy as a dynamic disease. 10.1007/978-3-662-05048-4. (Springer Berlin, Heidelberg, Berlin, 2003).
-
- Baud, M. O., Proix, T., Rao, V. R. & Schindler, K. Chance and risk in epilepsy. Curr. Opin. Neurol.33, 163–172 (2020). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

