Coordination of shoot apical meristem shape and identity by APETALA2 during floral transition in Arabidopsis
- PMID: 39138172
- PMCID: PMC11322546
- DOI: 10.1038/s41467-024-51341-6
Coordination of shoot apical meristem shape and identity by APETALA2 during floral transition in Arabidopsis
Abstract
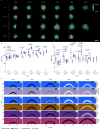
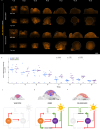
Plants flower in response to environmental signals. These signals change the shape and developmental identity of the shoot apical meristem (SAM), causing it to form flowers and inflorescences. We show that the increases in SAM width and height during floral transition correlate with changes in size of the central zone (CZ), defined by CLAVATA3 expression, and involve a transient increase in the height of the organizing center (OC), defined by WUSCHEL expression. The APETALA2 (AP2) transcription factor is required for the rapid increases in SAM height and width, by maintaining the width of the OC and increasing the height and width of the CZ. AP2 expression is repressed in the SAM at the end of floral transition, and extending the duration of its expression increases SAM width. Transcriptional repression by SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1) represents one of the mechanisms reducing AP2 expression during floral transition. Moreover, AP2 represses SOC1 transcription, and we find that reciprocal repression of SOC1 and AP2 contributes to synchronizing precise changes in meristem shape with floral transition.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

