Interplay between host humoral pattern recognition molecules controls undue immune responses against Aspergillus fumigatus
- PMID: 39138196
- PMCID: PMC11322389
- DOI: 10.1038/s41467-024-51047-9
Interplay between host humoral pattern recognition molecules controls undue immune responses against Aspergillus fumigatus
Abstract
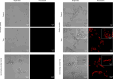
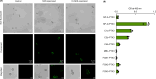
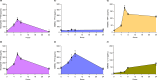
Pentraxin 3 (PTX3), a long pentraxin and a humoral pattern recognition molecule (PRM), has been demonstrated to be protective against Aspergillus fumigatus, an airborne human fungal pathogen. We explored its mode of interaction with A. fumigatus, and the resulting implications in the host immune response. Here, we demonstrate that PTX3 interacts with A. fumigatus in a morphotype-dependent manner: (a) it recognizes germinating conidia through galactosaminogalactan, a surface exposed cell wall polysaccharide of A. fumigatus, (b) in dormant conidia, surface proteins serve as weak PTX3 ligands, and (c) surfactant protein D (SP-D) and the complement proteins C1q and C3b, the other humoral PRMs, enhance the interaction of PTX3 with dormant conidia. SP-D, C3b or C1q opsonized conidia stimulated human primary immune cells to release pro-inflammatory cytokines and chemokines. However, subsequent binding of PTX3 to SP-D, C1q or C3b opsonized conidia significantly decreased the production of pro-inflammatory cytokines/chemokines. PTX3 opsonized germinating conidia also significantly lowered the production of pro-inflammatory cytokines/chemokines while increasing IL-10 (an anti-inflammatory cytokine) released by immune cells when compared to the unopsonized counterpart. Overall, our study demonstrates that PTX3 recognizes A. fumigatus either directly or by interplaying with other humoral PRMs, thereby restraining detrimental inflammation. Moreover, PTX3 levels were significantly higher in the serum of patients with invasive pulmonary aspergillosis (IPA) and COVID-19-associated pulmonary aspergillosis (CAPA), supporting previous observations in IPA patients, and suggesting that it could be a potential panel-biomarker for these pathological conditions caused by A. fumigatus.
© 2024. The Author(s).
Conflict of interest statement
All the authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
- ANR-21-CE17-0032-01 grant, FUNPOLYVAC/Agence Nationale de la Recherche (French National Research Agency)
- ANR-19-CE17-0021; BASIN/Agence Nationale de la Recherche (French National Research Agency)
- DEQ20150331722/Fondation pour la Recherche Médicale (Foundation for Medical Research in France)
- FungiNet 124/Deutsche Forschungsgemeinschaft (German Research Foundation)
- FungiNet 124/Deutsche Forschungsgemeinschaft (German Research Foundation)
LinkOut - more resources
Full Text Sources
Miscellaneous

