Progesterone (P4) ameliorates cigarette smoke-induced chronic obstructive pulmonary disease (COPD)
- PMID: 39138434
- PMCID: PMC11323532
- DOI: 10.1186/s10020-024-00883-y
Progesterone (P4) ameliorates cigarette smoke-induced chronic obstructive pulmonary disease (COPD)
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory lung disease associated with high morbidity and mortality worldwide. Oxidative injury and mitochondrial dysfunction in the airway epithelium are major events in COPD progression.
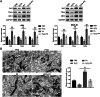
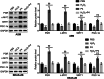
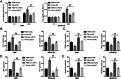
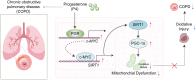
Methods and results: The therapeutic effects of Progesterone (P4) were investigated in vivo and in vitro in this study. In vivo, in a cigarette smoke (CS) exposure-induced COPD mouse model, P4 treatment significantly ameliorated CS exposure-induced physiological and pathological characteristics, including inflammatory cell infiltration and oxidative injury, in a dose-dependent manner. The c-MYC/SIRT1/PGC-1α pathway is involved in the protective function of P4 against CS-induced COPD. In vitro, P4 co-treatment significantly ameliorated H2O2-induced oxidative injury and mitochondrial dysfunctions by promoting cell proliferation, increasing mitochondrial membrane potential, decreasing ROS levels and apoptosis, and increasing ATP content. Moreover, P4 co-treatment partially attenuated H2O2-caused inhibition in Nrf1, Tfam, Mfn1, PGR-B, c-MYC, SIRT1, and PGC-1α levels. In BEAS-2B and ASM cells, the c-MYC/SIRT1 axis regulated P4's protective effects against H2O2-induced oxidative injury and mitochondrial dysfunctions.
Conclusion: P4 activates the c-MYC/SIRT1 axis, ameliorating CS-induced COPD and protecting both airway epithelial cells and smooth muscle cells against H2O2-induced oxidative damage. PGC-1α and downstream mitochondrial signaling pathways might be involved.
Keywords: Airway epithelium; Chronic obstructive pulmonary disease (COPD); Mitochondrial dysfunction; Oxidative injury; Progesterone (P4).
© 2024. The Author(s).
Conflict of interest statement
Not applicable.
Figures

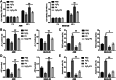

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

