Sigma-1 receptor exerts protective effects on ameliorating nephrolithiasis by modulating endoplasmic reticulum-mitochondrion association and inhibiting endoplasmic reticulum stress-induced apoptosis in renal tubular epithelial cells
- PMID: 39138590
- PMCID: PMC11328816
- DOI: 10.1080/13510002.2024.2391139
Sigma-1 receptor exerts protective effects on ameliorating nephrolithiasis by modulating endoplasmic reticulum-mitochondrion association and inhibiting endoplasmic reticulum stress-induced apoptosis in renal tubular epithelial cells
Abstract
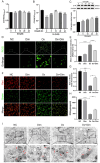
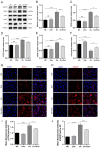
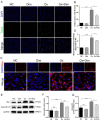
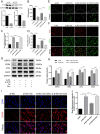
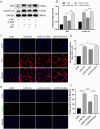
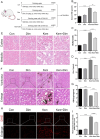
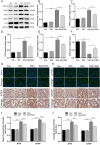
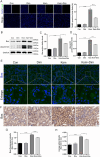
Oxalate-induced damage to renal tubular epithelial cells (RTECs) is an essential factor in the incident kidney stone, but the specific mechanism is unclear. Recent research has pinpointed interacting areas within the endoplasmic reticulum and mitochondria, called mitochondria-associated membranes (MAMs). These studies have linked endoplasmic reticulum stress (ERS) and oxidative imbalance to kidney disease development. The sigma-1 receptor (S1R), a specific protein found in MAMs, is involved in various physiological processes, but its role in oxalate-induced kidney stone formation remains unclear. In this study, we established cellular and rat models of oxalate-induced kidney stone formation to elucidate the S1R's effects against ERS and apoptosis and its mechanism in oxalate-induced RTEC injury. We found that oxalate downregulated S1R expression in RTECs and escalated oxidative stress and ERS, culminating in increased apoptosis. The S1R agonist dimemorfan up-regulated S1R expression and mitigated ERS and oxidative stress, thereby reducing apoptosis. This protective effect was mediated through S1R inhibition of the CHOP pathway. Animal experiments demonstrated that S1R's activation attenuated oxalate-induced kidney injury and alleviated kidney stone formation. This is the first study to establish the connection between S1R and kidney stones, suggesting S1R's protective role in inhibiting ERS-mediated apoptosis to ameliorate kidney stone formation.
Keywords: Sigmar-1 receptor; apoptosis; dimemorfan; endoplasmic reticulum stress; kidney stone; mitochondria-associated endoplasmic reticulum membrane; oxalate; oxidative stress.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
