Rationale and Design for the BLOCK-SAH Study (Pterygopalatine Fossa Block as an Opioid-Sparing Treatment for Acute Headache in Aneurysmal Subarachnoid Hemorrhage): A Phase II, Multicenter, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial with a Sequential Parallel Comparison Design
- PMID: 39138719
- PMCID: PMC11810580
- DOI: 10.1007/s12028-024-02078-z
Rationale and Design for the BLOCK-SAH Study (Pterygopalatine Fossa Block as an Opioid-Sparing Treatment for Acute Headache in Aneurysmal Subarachnoid Hemorrhage): A Phase II, Multicenter, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial with a Sequential Parallel Comparison Design
Abstract
Background: Acute post-subarachnoid hemorrhage (SAH) headaches are common and severe. Management strategies for post-SAH headaches are limited, with heavy reliance on opioids, and pain control is overall poor. Pterygopalatine fossa (PPF) nerve blocks have shown promising results in treatment of acute headache, including our preliminary and published experience with PPF-blocks for refractory post-SAH headache during hospitalization. The BLOCK-SAH trial was designed to assess the efficacy and safety of bilateral PPF-blocks in awake patients with severe headaches from aneurysmal SAH who require opioids for pain control and are able to verbalize pain scores.
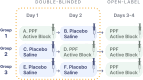
Methods: BLOCK-SAH is a phase II, multicenter, randomized, double-blinded, placebo-controlled clinical trial using the sequential parallel comparison design (SPCD), followed by an open-label phase.
Results: Across 12 sites in the United States, 195 eligible study participants will be randomized into three groups to receive bilateral active or placebo PPF-injections for 2 consecutive days with periprocedural monitoring of intracranial arterial mean flow velocities with transcranial Doppler, according to SPCD (group 1: active block followed by placebo; group 2: placebo followed by active block; group 3: placebo followed by placebo). PPF-injections will be delivered under ultrasound guidance and will comprise 5-mL injectates of 20 mg of ropivacaine plus 4 mg of dexamethasone (active PPF-block) or saline solution (placebo PPF-injection).
Conclusions: The trial has a primary efficacy end point (oral morphine equivalent/day use within 24 h after each PPF-injection), a primary safety end point (incidence of radiographic vasospasm at 48 h from first PPF-injection), and a primary tolerability end point (rate of acceptance of second PPF-injection following the first PPF-injection). BLOCK-SAH will inform the design of a phase III trial to establish the efficacy of PPF-block, accounting for different headache phenotypes.
Trial registration: ClinicalTrials.gov NCT06008795.
Keywords: Headache; Intensive care; Nerve block; Opioid analgesics; Subarachnoid hemorrhage.
© 2024. The Author(s).
Conflict of interest statement
Conflict of interest: CBM has received honoraria by the American Academy of Neurology for speaking and writing. KMB has received compensation as an associate editor for Critical Care Medicine and reports honoraria by the American Academy of Neurology for speaking and course directorship. Ethical Approval/Informed Consent: This protocol has been approved by the central institutional review board (Advarra) for this trial, and by the trial’s Data Safety and Monitoring Board. Trial Registration: ClinicalTrials.gov, NCT06008795. Registered 18 August 2023, https://clinicaltrials.gov/study/NCT06008795 .
Figures


References
-
- Maciel CB, Barlow B, Lucke-Wold B, Gobinathan A, Abu-Mowis Z, Peethala MM, et al. Acute headache management for patients with subarachnoid hemorrhage: an international survey of health care providers. Neurocrit Care. 2023;38(2):395–406. - PubMed
-
- Morad AH, Tamargo RJ, Gottschalk A. The longitudinal course of pain and analgesic therapy following aneurysmal subarachnoid hemorrhage: a cohort study. Headache. 2016;56(10):1617–25. - PubMed
-
- Viswanathan V, Lucke-Wold B, Jones C, Aiello G, Li Y, Ayala A, et al. Change in opioid and analgesic use for headaches after aneurysmal subarachnoid hemorrhage over time. Neurochirurgie. 2021;67(5):427–32. - PubMed
-
- Bouchier B, Demarquay G, Dailler F, Lukaszewicz AC, Ritzenthaler T. Course of headaches and predictive factors associated with analgesia failure following spontaneous subarachnoid hemorrhage: a prospective cohort study. J Neurosurg Anesthesiol. 2023;35(3):333–7. - PubMed
-
- Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G, European Stroke Organization European Stroke. Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 2013;35(2):93–112. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

