miR-92a-3p and miR-320a are Upregulated in Plasma Neuron-Derived Extracellular Vesicles of Patients with Frontotemporal Dementia
- PMID: 39138758
- PMCID: PMC11772464
- DOI: 10.1007/s12035-024-04386-z
miR-92a-3p and miR-320a are Upregulated in Plasma Neuron-Derived Extracellular Vesicles of Patients with Frontotemporal Dementia
Abstract
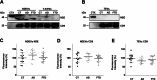
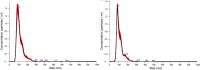
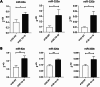
Despite the efforts to identify fluid biomarkers to improve diagnosis of Frontotemporal dementia (FTD), only a few candidates have been described in recent years. In a previous study, we identified three circulating miRNAs (miR-92a-3p, miR-320a and miR-320b) differentially expressed in FTD patients with respect to healthy controls and/or Alzheimer's disease (AD) patients. Now, we investigated whether those changes could be due to miRNAs contained in neuron-derived extracellular vesicles (NDEVs). We also evaluated miRNAs content in total plasma EVs and in CSF samples. The analysis of plasma NDEVs carried out on 40 subjects including controls (n = 13), FTD (n = 13) and AD (n = 14) patients, showed that both miR-92a-3p and miR-320a levels were triplicated in the FTD group if compared with CT and AD patients. Increased levels of the same miRNAs were found also in CSF derived from FTD group compared to CTs. No differences were observed in expression levels of miR-320b among the three groups. Worthy of note, all miRNAs analysed were increased in an FTD cell model, MAPT IVS10 + 16 neurons. Our results suggest that miR-92a and miR-320a in NDEVs could be proposed as FTD biomarkers.
Keywords: Alzheimer’s disease; Extracellular vesicles; Frontotemporal dementia; Human iPSCs; MicroRNA.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing Interests: PP and RR report being employed by Istituto Superiore di Sanità, GB reports being employed by the University of Rome “Sapienza,” and MAD report being employed by the University of Trento, the three research institutions having a joined patent application pending on the findings described in the present article. PP, GB, RR, and MAD are co-inventors on this patent and, as such, are entitled to a share of potential royalties The remaining authors have no competing interests to declare.
Figures



References
-
- Braak H, Braak E (1997) Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 18:351–357. 10.1016/s0197-4580(97)00056-0 - PubMed
-
- Cardarelli R, Kertesz A, Knebl JA (2010) Frontotemporal dementia: a review for primary care physicians. Am Fam Physician 82:1372–1377 - PubMed
-
- Seltman RE, Matthews BR (2012) Frontotemporal lobar degeneration: epidemiology, pathology, diagnosis, and management. CNS Drugs 26:841–870. 10.2165/11640070-000000000-00000 - PubMed
-
- Tartaglia MC, Mackenzie IRA (2023) Recent advances in frontotemporal dementia. Can J Neurol Sc 50:485–494. 10.1017/cjn.2022.69 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

