The 'Prostate Embolisation AS first-line therapY compAred to meDication in treatment naïVe men with prostAte eNlargement, a randomised ControllEd trial' (P-EASY ADVANCE): a randomised controlled trial of prostate embolisation vs medication for BPH
- PMID: 39139009
- PMCID: PMC11603100
- DOI: 10.1111/bju.16479
The 'Prostate Embolisation AS first-line therapY compAred to meDication in treatment naïVe men with prostAte eNlargement, a randomised ControllEd trial' (P-EASY ADVANCE): a randomised controlled trial of prostate embolisation vs medication for BPH
Abstract
Objective: To compare prostate artery embolisation (PAE) to the combination of tamsulosin and dutasteride therapy as a potential first-line therapy for obstructive benign prostatic hyperplasia (BPH) in treatment-naïve patients in the 'Prostate Embolisation AS first-line therapY compAred to meDication in treatment naïVe men with prostAte eNlargement, a randomised ControllEd trial' (P-EASY ADVANCE).
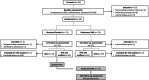
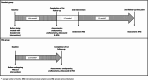
Patients and methods: A total of 39 men with enlarged prostates, moderate-severe lower urinary tract symptoms (LUTS) and obstructed/equivocal urodynamic studies (UDS), and who had no prior treatment for BPH, were randomised to receive either combined medical therapy with tamsulosin and dutasteride (medication) or PAE. Follow-up UDS, International Prostate Symptom Score (IPSS), uroflowmetry and ultrasound were performed at short- to medium-term intervals following interventions and compared to baseline.
Results: The medication and PAE treatment groups had similar baseline characteristics, including prostate volumes (87.8 and 85.4 mL respectively), maximum urinary flow rate (Qmax; 6.5 and 6.6 mL/s, respectively), IPSS (19.5 and 21, respectively) and obstructed UDS (79% and 74%, respectively). Both interventions improved voiding and bladder outflow obstruction from baseline, with more patients unobstructed after PAE (63%) compared to medication (28%) (P = 0.03). PAE patients had significantly greater reductions in prostate size (P < 0.001), incomplete emptying (P = 0.002), total IPSS (P = 0.032), Qmax (P = 0.006) and quality of life (P = 0.001). Altered ejaculation, erectile dysfunction and nausea were more common in the medication group.
Conclusion: Prostate artery embolisation was more effective than combined medical therapy at reducing urinary obstruction, decreasing prostate volume and improving LUTS in patients with BPH who had not previously been treated. This is the first randomised control study to compare PAE and combined medical therapy in exclusively treatment-naïve patients and raises the potential of PAE as an alternative early treatment option for BPH. Further randomised comparative trials are planned to further validate the role of PAE in mitigating obstructive BPH.
Keywords: benign prostatic hyperplasia; embolisation; medical therapy; urinary tract symptoms; urodynamics.
© 2024 The Author(s). BJU International published by John Wiley & Sons Ltd on behalf of BJU International.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Madersbacher S, Marszalek M, Lackner J, Berger P, Schatzl G. The long‐term outcome of medical therapy for BPH. Eur Urol 2007; 51: 1522–1533 - PubMed
-
- Pisco JM, Bilhim T, Pinheiro LC et al. Medium‐ and long‐term outcome of prostate artery embolization for patients with benign prostatic hyperplasia: results in 630 patients. J Vasc Interv Radiol 2016; 27: 1115–1122 - PubMed
-
- Bilhim T, Costa NV, Torres D, Pinheiro LC, Spaepen E. Long‐term outcome of prostatic artery embolization for patients with benign prostatic hyperplasia: single‐centre retrospective study in 1072 patients over a 10‐year period. Cardiovasc Intervent Radiol 2022; 45: 1324–1336 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

