To target cellular senescence in diabetic kidney disease: the known and the unknown
- PMID: 39139135
- PMCID: PMC11327223
- DOI: 10.1042/CS20240717
To target cellular senescence in diabetic kidney disease: the known and the unknown
Abstract
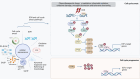
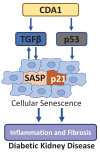
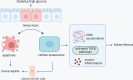
Cellular senescence represents a condition of irreversible cell cycle arrest, characterized by heightened senescence-associated beta-galactosidase (SA-β-Gal) activity, senescence-associated secretory phenotype (SASP), and activation of the DNA damage response (DDR). Diabetic kidney disease (DKD) is a significant contributor to end-stage renal disease (ESRD) globally, with ongoing unmet needs in terms of current treatments. The role of senescence in the pathogenesis of DKD has attracted substantial attention with evidence of premature senescence in this condition. The process of cellular senescence in DKD appears to be associated with mitochondrial redox pathways, autophagy, and endoplasmic reticulum (ER) stress. Increasing accumulation of senescent cells in the diabetic kidney not only leads to an impaired capacity for repair of renal injury, but also the secretion of pro-inflammatory and profibrotic cytokines and growth factors causing inflammation and fibrosis. Current treatments for diabetes exhibit varying degrees of renoprotection, potentially via mitigation of senescence in the diabetic kidney. Targeting senescent cell clearance through pharmaceutical interventions could emerge as a promising strategy for preventing and treating DKD. In this paper, we review the current understanding of senescence in DKD and summarize the possible therapeutic interventions relevant to senescence in this field.
Keywords: Cellular Senescence; diabetic nephropathy; inlfammation; renal fibrosis.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

