Canadian colorectal cancer screening programs: How do they measure up using the International Agency for Research on Cancer criteria for organized screening?
- PMID: 39139223
- PMCID: PMC11317627
- DOI: 10.1093/jcag/gwae015
Canadian colorectal cancer screening programs: How do they measure up using the International Agency for Research on Cancer criteria for organized screening?
Abstract
Background: Canada has one of the highest incidences of colorectal cancer (CRC) worldwide. CRC screening improves CRC outcomes and is cost-effective. This study compares Canadian CRC screening programs using essential elements of an organized screening program outlined by the International Agency for Research on Cancer (IARC).
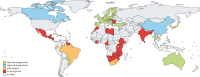
Methods: We collaborated with the Cancer Screening in 5 continents (CanScreen5) program, an initiative of IARC. Standardized data collection forms were sent to representatives of provincial and territorial CRC screening programs. Twenty-five questions were selected to reflect IARC's essential elements of an organized screening program. We performed a qualitative analysis of Canada's CRC screening programs and compared programs within Canada and internationally.
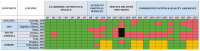
Results: CRC screening programs exist in 10 provinces and 2 territories. None of the programs in Canada met all the essential criteria of an organized screening program outlined by IARC. Three programs do not send invitations to participate in screening. Among those that do, 4 programs do not include a stool test kit in the invitations. While all provinces met the essential elements for leadership, governance, finance, and access to essential services, there was more heterogeneity in the domains of service delivery as well as information systems and quality assurance.
Conclusions: There is considerable heterogeneity in the design of CRC screening programs in Canada and worldwide. Programs should strive to meet all the essential IARC criteria for organized screening if local resources allow, such as issuing invitations and implementing systems to track and compare outcomes to maximize screening program quality, effectiveness, and impact.
Keywords: Colorectal cancer; organized; screening.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Canadian Association of Gastroenterology.
Conflict of interest statement
C.C.Y.L., L.Z., L.R., A.N.B., D.L., D.M., E.K., N.N.B., J.M., L.C., D.S., K.E., K.B., B.R.M., M.K., R.S., S.A., S.P., S.J.H., T.Z., S.E., J.K., M.M., P.B.: None; A.L.C. is the recipient of grants or contracts from the European Commission, WHO-EURO, and the American Cancer Society; A.N.K. is supported by the Canadian Partnership Against Cancer; D.A. has served as an expert advisor to the Canadian Partnership Against Cancer and has received travel and accommodation support for CPAC meetings; C.K.W. serves as Section Chief at Alberta Health Services and as an academic staff at the University of Alberta; C.D. is contracted by Cancer Care Ontario/Ontario Health as Clinical lead for the ColonCancerCheck program. The C.D. serves on the Data Safety monitoring board for the COOP trial (colonoscopy vs stool-based testing for older adults with a history of colon polyps) and as medical lead for the Champlain region GI endoscopy central intake program; J.T. has received grants or contracts from BC Cancer and BC Ministry of Health; E.K. serves on the Board of Directors of the Canadian Cancer Society and the Canadian Association of Provincial Cancer Agencies; H.S. is the recipient of an Investigator Initiated Grant from Pfizer Canada. He has also received consulting fees from Pendopharm Canada, Ferring Canada, Amgen Canada, Sandoz Canada, Takeda Canada, Bristol-Myers Squibb Canada, Guardant Health, and Abbvie Canada. He also serves on committees in the Canadian Association of Gastroenterology; H.D.P. has received support from the Saskatchewan Medical Association CME fund to attend meetings; L.G. has received support from the Canadian Partnership Against Cancer to attend network member meetings; M.H.G. received support to attend an education event held by Société de Radiologie du Québec; M.S. has clinical trial sponsorships from Theravance, Hoffmann-La Roche, Abbive, and Janseen. M.S. has served as a consultant for Celltrion, Eli Lilly, Amgen, Bristol-Myers-Squib, Sandoz, Pfizer, Jannsen, Takeda, and Abbvie. M.S. has received speaker fees from Eli Lilly, Abbvie, Takeda, and Janseen; J.T. serves as the lead scientist of the Ontario Health ColonCancerCheck program and as the Provincial Medical Dirctor of Cancer Control.
Figures

References
-
- Brenner DR, Poirier A, Woods RR, et al. Projected estimates of cancer in Canada in 2022. Can Med Assoc J [Internet]. 2022;194(17):E601 LP-E607. Available from: http://www.cmaj.ca/content/194/17/E601.abstract - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
