Coupling of droplet-on-demand microfluidcs with ESI/MS to study single-cell catalysis
- PMID: 39139235
- PMCID: PMC11320962
- DOI: 10.1039/d4ra04835k
Coupling of droplet-on-demand microfluidcs with ESI/MS to study single-cell catalysis
Abstract
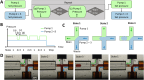
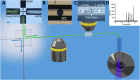
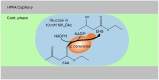
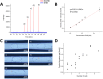
Droplet microfluidics provides an efficient method for analysing reactions within the range of nanoliters to picoliters. However, the sensitive, label-free and versatile detection with ESI/MS poses some difficulties. One challenge is the difficult association of droplets with the MS signal in high-throughput droplet analysis. Hence, a droplet-on-demand system for the generation of a few droplets can address this and other problems such as surfactant concentration or cross-contamination. Accordingly, the system has been further developed for online coupling with ESI/MS. To achieve this, we developed a setup enabling on-demand droplet generation by hydrodynamic gating, with downstream microscopic droplet detection and MS analysis. This facilitated the incorporation of 1-9 yeast cells into individual 1-5 nL droplets and the monitoring of yeast-catalysed transformation from ketoester to ethyl-3-hydroxybutyrate by MS. With our method a mean production rate of 0.035 ± 0.017 fmol per cell per h was observed with a detection limit of 0.30 μM. In conclusion, our droplet-on-demand method is a versatile and advantageous tool for cell encapsulation in droplets, droplet imaging and reaction detection using ESI/MS.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Oliveira A. F. Pessoa A. C. S. N. Bastos R. G. de La Torre L. G. Microfluidic tools toward industrial biotechnology. Biotechnol. Prog. 2016;32:1372–1389. - PubMed
-
- Zhu P. Wang L. Passive and active droplet generation with microfluidics: a review. Lab Chip. 2016;17:34–75. - PubMed
-
- Sjostrom S. L. Bai Y. Huang M. Liu Z. Nielsen J. Joensson H. N. Andersson Svahn H. High-throughput screening for industrial enzyme production hosts by droplet microfluidics. Lab Chip. 2014;14:806–813. - PubMed
LinkOut - more resources
Full Text Sources

