Surgical treatment of refractory low back pain using implanted BurstDR spinal cord stimulation (SCS) in a cohort of patients without options for corrective surgery: Findings and results from the DISTINCT study, a prospective randomized multi-center-controlled trial
- PMID: 39139617
- PMCID: PMC11321325
- DOI: 10.1016/j.xnsj.2024.100508
Surgical treatment of refractory low back pain using implanted BurstDR spinal cord stimulation (SCS) in a cohort of patients without options for corrective surgery: Findings and results from the DISTINCT study, a prospective randomized multi-center-controlled trial
Abstract
Background: Low back pain (LBP) is a highly prevalent, disabling condition affecting millions of people. Patients with an identifiable anatomic pain generator and resulting neuropathic lower extremity symptoms often undergo spine surgery, but many patients lack identifiable and/or surgically corrective pathology. Nonoperative treatment options often fail to provide sustained relief. Spinal cord stimulation (SCS) is sometimes used to treat these patients, but the lack of level 1 evidence limits its widespread use and insurance coverage. The DISTINCT RCT study evaluates the efficacy of passive recharge burst SCS compared to conventional medical treatment (CMM) in alleviating chronic, refractory axial low back pain.
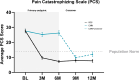
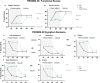
Methods: This prospective, multicenter, randomized, study with an optional 6-month crossover involved patients who were not candidates for lumbar spine surgery. The primary and secondary endpoints evaluated improvements in low back pain intensity (NRS), back pain-related disability (ODI), pain catastrophizing (PCS), and healthcare utilization. Patients were randomized to SCS therapy or CMM at 30 US study sites.
Results: The SCS arm reported an 85.3% NRS responder rate (≥ 50% reduction) compared to 6.2% (5/81) in the CMM arm. After the 6M primary endpoint, SCS patients elected to remain on assigned therapy and 66.2% (49/74) of CMM patients chose to trial SCS (crossover). At the 12M follow-up, SCS and crossover patients reported 78.6% and 71.4% NRS responder rates. Secondary outcomes indicated significant improvements in ODI, PCS, and reduced healthcare utilization. Six serious adverse events were reported and resolved without sequelae.
Conclusion: DISTINCT chronic low back pain patients with no indication for corrective surgery experienced a significant and sustained response to burst SCS therapy for up to 12 months. CMM patients who crossed over to the SCS arm reported profound improvements after 6 months. This data advocates for a timely consideration of SCS therapy in patients unresponsive to conservative therapy.
Keywords: BurstDR; Chronic low back pain burst; DISTINCT RCT; Nonsurgical Low back pain; Passive recharge burst; SCS.
© 2024 The Author(s).
Conflict of interest statement
One or more of the authors declare financial or professional relationships on ICMJE-NASSJ disclosure forms.
Figures



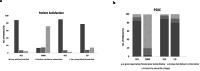
References
-
- Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. Lancet. 2018;391(10137):2356–2367. - PubMed
-
- Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64(6):2028–2037. - PubMed
-
- Hoy D, March L, Brooks P, et al. Measuring the global burden of low back pain. Best Pract Res Clin Rheumatology. 2010;24(2):155–165. - PubMed
-
- Panjabi MM. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. Journal of spinal disorders. 1992;5(4):383–389. discussion 397. - PubMed
-
- Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8(1):8–20. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

