Optical constraints on two-photon voltage imaging
- PMID: 39139631
- PMCID: PMC11321468
- DOI: 10.1117/1.NPh.11.3.035007
Optical constraints on two-photon voltage imaging
Abstract
Significance: Genetically encoded voltage indicators (GEVIs) are a valuable tool for studying neural circuits in vivo, but the relative merits and limitations of one-photon (1P) versus two-photon (2P) voltage imaging are not well characterized.
Aim: We consider the optical and biophysical constraints particular to 1P and 2P voltage imaging and compare the imaging properties of commonly used GEVIs under 1P and 2P excitation.
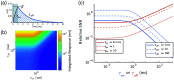
Approach: We measure the brightness and voltage sensitivity of voltage indicators from commonly used classes under 1P and 2P illumination. We also measure the decrease in fluorescence as a function of depth in the mouse brain. We develop a simple model of the number of measurable cells as a function of reporter properties, imaging parameters, and desired signal-to-noise ratio (SNR). We then discuss how the performance of voltage imaging would be affected by sensor improvements and by recently introduced advanced imaging modalities.
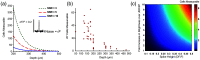
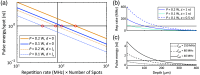
Results: Compared with 1P excitation, 2P excitation requires -fold more illumination power per cell to produce similar photon count rates. For voltage imaging with JEDI-2P in the mouse cortex with a target SNR of 10 (spike height to baseline shot noise), a measurement bandwidth of 1 kHz, a thermally limited laser power of 200 mW, and an imaging depth of , 2P voltage imaging using an 80-MHz source can record from no more than neurons simultaneously.
Conclusions: Due to the stringent photon-count requirements of voltage imaging and the modest voltage sensitivity of existing reporters, 2P voltage imaging in vivo faces a stringent tradeoff between shot noise and tissue photodamage. 2P imaging of hundreds of neurons with high SNR at a depth of will require either major improvements in 2P GEVIs or qualitatively new approaches to imaging.
Keywords: shot noise; two-photon; voltage imaging.
© 2024 The Authors.
Figures



Update of
-
Optical constraints on two-photon voltage imaging.bioRxiv [Preprint]. 2024 May 17:2023.11.18.567441. doi: 10.1101/2023.11.18.567441. bioRxiv. 2024. Update in: Neurophotonics. 2024 Jul;11(3):035007. doi: 10.1117/1.NPh.11.3.035007. PMID: 38014011 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources

