Sex-Dependent Effects of Angiotensin Type 2 Receptor-Expressing Medial Prefrontal Cortex Interneurons in Fear Extinction Learning
- PMID: 39140003
- PMCID: PMC11321323
- DOI: 10.1016/j.bpsgos.2024.100340
Sex-Dependent Effects of Angiotensin Type 2 Receptor-Expressing Medial Prefrontal Cortex Interneurons in Fear Extinction Learning
Abstract
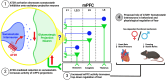
Background: The renin-angiotensin system has been identified as a potential therapeutic target for posttraumatic stress disorder, although its mechanisms are not well understood. Brain angiotensin type 2 receptors (AT2Rs) are a subtype of angiotensin II receptors located in stress and anxiety-related regions, including the medial prefrontal cortex (mPFC), but their function and mechanism in the mPFC remain unexplored. Therefore, we used a combination of imaging, cre/lox, and behavioral methods to investigate mPFC-AT2R-expressing neurons in fear and stess related behavior.
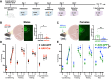
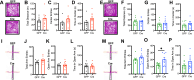
Methods: To characterize mPFC-AT2R-expressing neurons in the mPFC, AT2R-Cre/tdTomato male and female mice were used for immunohistochemistry. mPFC brain sections were stained with glutamatergic or interneuron markers, and density of AT2R+ cells and colocalization with each marker were quantified. To assess fear-related behaviors in AT2R-flox mice, we selectively deleted AT2R from mPFC neurons using a Cre-expressing adeno-associated virus. Mice then underwent Pavlovian auditory fear conditioning, elevated plus maze, and open field testing.
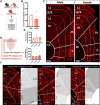
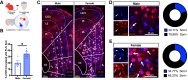
Results: Immunohistochemistry results revealed that AT2R was densely expressed throughout the mPFC and primarily expressed in somatostatin interneurons in a sex-dependent manner. Following fear conditioning, mPFC-AT2R Cre-lox deletion impaired extinction and increased exploratory behavior in female but not male mice, while locomotion was unaltered by mPFC-AT2R deletion in both sexes.
Conclusions: These results identify mPFC-AT2R+ neurons as a novel subgroup of somatostatin interneurons and reveal their role in regulating fear learning in a sex-dependent manner, potentially offering insights into novel therapeutic targets for posttraumatic stress disorder.
Keywords: AT2R; Angiotensin II; Fear extinction; Interneuron; PTSD; Prefrontal cortex.
Plain language summary
Posttraumatic stress disorder (PTSD) is a significant predictor of cardiovascular disease (CVD), although the underlying mechanisms are poorly understood. The brain renin-angiotensin system (RAS) is important for cardiovascular and emotional stress regulation and may better help understand the link between PTSD and CVD risk. Our research reveals that the brain angiotensin II type 2 receptor (AT2R) subtype is located on specific somatostatin (SOM+) interneurons in the medial prefrontal cortex (mPFC) and plays a role in fear memory extinction, particularly in females. These findings reveal a role for the mPFC-AT2R in fear-based learning and memory, offering potential insights into the mechanisms underlying the PTSD-CVD association and therapeutic strategies.
© 2024 The Authors.
Figures


References
-
- Kimerling R., Allen M.C., Duncan L.E. Chromosomes to social contexts: Sex and gender differences in PTSD. Curr Psychiatry Rep. 2018;20:114. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

