Development of Novel Bacterial Topoisomerase Inhibitors Assisted by Computational Screening
- PMID: 39140037
- PMCID: PMC11318591
- DOI: 10.1021/acsmedchemlett.4c00162
Development of Novel Bacterial Topoisomerase Inhibitors Assisted by Computational Screening
Abstract
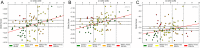
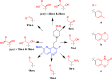
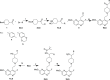
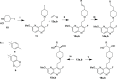
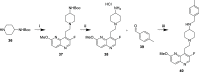
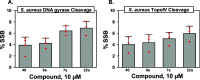
Multidrug-resistant bacterial infections pose an ever-evolving threat to public health. Since the outset of the antibacterial age, bacteria have developed a multitude of diverse resistance mechanisms that suppress the effectiveness of current therapies. New drug entities, such as Novel Bacterial Topoisomerase Inhibitors (NBTIs), can circumvent this major issue. A computational docking model was employed to predict the binding to DNA gyrase of atypical NBTIs with novel pharmacophores. Synthesis of NBTIs based on computational docking and subsequent antibacterial evaluation against both Gram-positive and Gram-negative bacteria yielded congeners with outstanding anti-staphylococcal activity and varying activity against select Gram-negative pathogens.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): MMF is a shareholder of Pfizer.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

