TRPA1 Inhibition Effects by 3-Phenylcoumarin Derivatives
- PMID: 39140042
- PMCID: PMC11318103
- DOI: 10.1021/acsmedchemlett.4c00072
TRPA1 Inhibition Effects by 3-Phenylcoumarin Derivatives
Abstract
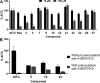
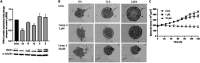
Transient receptor potential ankyrin 1 (TRPA1) protein plays an important role in the inflammatory response, and it has been associated with different pain conditions and pain-related diseases, making TRPA1 a valid target for painkillers. In this study, we identified potential TRPA1 inhibitors and located their binding sites utilizing computer-aided drug design (CADD) techniques. The designed 3-phenylcoumarin-based TRPA1 inhibitors were successfully synthesized using a microwave assisted synthetic strategy. 3-(3-Bromophenyl)-7-acetoxycoumarin (5), 7-hydroxy-3-(3-hydroxyphenyl)coumarin (12) and 3-(3-hydroxyphenyl)coumarin (23) all showed inhibitory activity toward TRPA1 in vitro. Compound 5 also decreased the size and formation of breast cancer cells. Hence, targeting TRPA1 may represent a promising alternative for the treatment of pain and inflammation.
© 2024 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
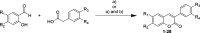

References
-
- Timonen J. M.; Vuolteenaho K.; Leppänen T.; Nieminen R. M.; Aulaskari P.; Jänis J.; Vainiotalo P.; Moilanen E. Synthesis of Novel Anti-inflammatory Psoralen Derivatives - Structures with Distinct Anti-Inflammatory Activities. J. Heterocycl. Chem. 2018, 55 (11), 2590–2597. 10.1002/jhet.3318. - DOI
-
- Finnerup N. B.; Haroutounian S.; Kamerman P.; Baron R.; Bennett D. L. H.; Bouhassira D.; Cruccu G.; Freeman R.; Hansson P.; Nurmikko T.; Raja S. N.; Rice A. S. C.; Serra J.; Smith B. H.; Treede R. D.; Jensen T. S. Neuropathic pain: an updated grading system for research and clinical practice. Pain 2016, 157 (8), 1599–1606. 10.1097/j.pain.0000000000000492. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

