Comprehensive Geriatric Assessment to Optimize the Management of Older Patients With Transthyretin Cardiac Amyloidosis
- PMID: 39140080
- PMCID: PMC11318635
- DOI: 10.1016/j.jacadv.2024.101123
Comprehensive Geriatric Assessment to Optimize the Management of Older Patients With Transthyretin Cardiac Amyloidosis
Abstract
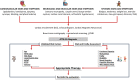
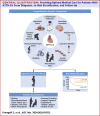
Transthyretin cardiac amyloidosis (ATTR-CA) predominantly affects older adults with multiple chronic conditions, leading to significant physical, cognitive, and emotional challenges. New disease-modifying drugs are effective in early stages, prompting a shift toward comprehensive assessments, including functional capacity and quality of life. However, these assessments may not fully capture the complexity of older ATTR-CA patients, especially regarding frailty and mood disorders, which can influence symptom reporting. Thus, integrating comprehensive geriatric assessment tools into routine clinical practice may be crucial to detect early signs of frailty or functional impairment that could impact outcomes and mitigate futility and ageism in the decision-making process. This review highlights the importance of evaluating multimorbidity, disability, and frailty in older patients with ATTR-CA to optimize management strategies.
Keywords: cardiac amyloidosis; comprehensive geriatric assessment; frailty; prognosis; risk stratification.
© 2024 The Authors.
Conflict of interest statement
Dr Maurer is supported by grant from 10.13039/100000002NIH R01HL139671 and R01AG081582-01; grants and personal fees from Alnylam, Pfizer, Eidos, Prothena, and Ionis; and personal fees from AstraZeneca, Akcea, Intellia, and Novo Nordisk. Dr Fontana is supported by a British Heart Foundation Intermediate Clinical Research Fellowship (FS/18/21/33447). Dr Gillmore has received consultancy fees from Alnylam, AstraZeneca, ATTRalus, Bridgebio, Ionis, Intellia, Lycia, and Pfizer. Dr Goyal is supported by 10.13039/100000049National Institute on Aging grants K76AG064428, R21AG077092, R01AG085420, and R24AG064025; and has received consulting fees from Agepha Pharma, Akros Pharma, Axon therapies, and Sensorum Health. Dr Fine has received research support and consulting honoraria from Pfizer, Alnylam, Ionis, AstraZeneca, Novo Nordisk, Sanofi/Genzyme, and Eidos/BridgeBio. Dr Grogan has received research support and or advisory board/consulting fees from Alnylam, BridgeBio/Eidos, Janssen, Pfizer, AstraZeneca, Novo, and Nordisk (all funds paid to employer, no personal compensation). Dr Marfella is supported by Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale (Scientific research programs of high national interest) # 2020LM8WNW. Dr Limongelli has received research support and or advisory board/consulting fees from Alnylam, Pfizer, and AstraZeneca. Dr Longhi is supported by the Italian Ministry of Health, RC-2022-2773270 project. Dr Cuddy is supported by grant support from 10.13039/100000002NIH 1K23HL166686-01 and 10.13039/100000967AHA 23CDA857664; and has received personal fees from Pfizer, Bridgebio, Ionis, AstraZeneca, and Novo Nordisk. Dr Masri is supported by Research Grants from 10.13039/100004319Pfizer, Ionis, Attralus, and 10.13039/100014941Cytokinetics; and has received fees from Cytokinetics, BMS, Eidos, Pfizer, Ionis, Lexicon, Attralus, Haya, BioMarin, Alexion, and Tenaya. Dr Olivotto is supported by grants for “Respect,” “StratifyHF,” and “SmashHCM” and has received grants from 10.13039/100015340Bayer, 10.13039/100016619MyoKardia, Inc, which is a wholly owned subsidiary of Bristol Myers Squibb, Sanofi Genzyme, and Shire, which is now part of Takeda; personal fees from Bayer, Sanofi Genzyme, and Shire/Takeda; and payments as a consultant from MyoKardia, Inc. Dr Perfetto has received honoraria for advisory board participation from Pfizer, Alnylam, and Akcea. Dr Ungar is supported by Piano Nazionale di Ripresa e Resilienza grants. Dr Cappelli has received consultant fees from Pfizer, Alnylam, AstraZeneca, BridgeBio, Daiichi Sankyo, and Novo Nordisk. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures


References
-
- Rapezzi C., Lorenzini M., Longhi S., et al. Cardiac amyloidosis: the great pretender. Heart Fail Rev. 2015;20:117–124. - PubMed
-
- Garcia-Pavia P., Rapezzi C., Adler Y., et al. Diagnosis and treatment of cardiac amyloidosis. A position statement of the European Society of cardiology working Group on Myocardial and Pericardial diseases. Eur J Heart Fail. 2021;23:512–526. - PubMed
-
- Zampieri M., Nardi G., Del Monaco G., et al. Changes in the perceived epidemiology of amyloidosis: 20 year-experience from a Tertiary referral Centre in Tuscany. Int J Cardiol. 2021;335:123–127. - PubMed
-
- Fumagalli C., Zampieri M., Perfetto F., et al. Early diagnosis and outcome in patients with wild-type transthyretin cardiac amyloidosis. Mayo Clin Proc. 2021;96:2185–2191. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

