Treatment characteristics and outcomes in lower-risk, non-del(5q) myelodysplastic syndromes: findings from a medical record review in the USA, Canada and Europe
- PMID: 39140298
- PMCID: PMC11497946
- DOI: 10.1080/14796694.2024.2379228
Treatment characteristics and outcomes in lower-risk, non-del(5q) myelodysplastic syndromes: findings from a medical record review in the USA, Canada and Europe
Abstract
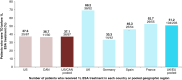
Aim: To assess treatment patterns and outcomes in patients with non-del(5q) lower-risk myelodysplastic syndromes.Methods: Patient medical records were reviewed in the USA, Canada (CAN), UK and the EU.Results: Analysis included 119 patients in the USA/CAN (median age, 61.5 years) and 245 patients in the UK/EU (median age, 67.3 years). Most patients received erythropoiesis-stimulating agents (ESAs) as first-line (1L) therapy (USA/CAN: 89.0%; UK/EU: 90.2%). A substantial proportion of 1L erythropoiesis-stimulating agent-treated patients were transfusion dependent before 1L (USA/CAN: 37.1%; UK/EU: 51.2%); a small percentage of these patients achieved transfusion independence during 1L therapy (USA/CAN: 2.8%; UK/EU: 14.4%).Conclusion: These findings highlight an unmet need for more effective treatments among patients with non-del(5q) lower-risk myelodysplastic syndromes.
Keywords: hematologic outcomes; lower-risk myelodysplastic syndromes; non-del(5q); real-world data; treatment patterns.
Plain language summary
[Box: see text].
Conflict of interest statement
RK Goyal, RC Parikh, E Esterberg, M Jimenez and M Sluga-O'Callaghan are full-time employees of RTI Health Solutions, an independent nonprofit research organization that was retained by Bristol Myers Squibb to conduct the research that is the subject of this manuscript. Their compensation is unconnected to the studies on which they work. A Yucel, D Miteva and H Xiao are employees of Bristol Myers Squibb and may hold shares and/or stock options in the company. M Diez-Campelo is a full-time employee of University Hospital of Salamanca, Salamanca, Spain and has received speaker honoraria from Bristol Myers Squibb and Novartis, and served on advisory boards for Agios, Blueprint Medicines, Bristol Myers Squibb, GlaxoSmithKline, Hemavant, Keros, Novartis, and Syros, and has received support for attending meetings and/or travel from Gilead. U Germing is a full-time employee of the University Hospital of Dusseldorf, Dusseldorf, Germany, and has received speaker honoraria from Bristol Myers Squibb, Janssen and Novartis, and consulting fees from Bristol Myers Squibb, as well as grants or contracts from Bristol Myers Squibb and Novartis. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures


References
-
- Almeida A, Fenaux P, List AF, et al. . Recent advances in the treatment of lower-risk non-del(5q) myelodysplastic syndromes (MDS). Leuk Res. 2017;52:50–57. doi:10.1016/j.leukres.2016.11.008 - DOI - PubMed
-
•• An overview of the current treatment options for lower-risk non-del(5q) myelodysplastic syndromes (MDS).
-
- de Swart L, Smith A, Johnston TW, et al. . Validation of the revised international prognostic scoring system (IPSS-R) in patients with lower-risk myelodysplastic syndromes: a report from the prospective European LeukaemiaNet MDS (EUMDS) registry. Br J Haematol. 2015;170(3):372–383. doi:10.1111/bjh.13450 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
