Antibody characterization is critical to enhance reproducibility in biomedical research
- PMID: 39140332
- PMCID: PMC11324233
- DOI: 10.7554/eLife.100211
Antibody characterization is critical to enhance reproducibility in biomedical research
Abstract
Antibodies are used in many areas of biomedical and clinical research, but many of these antibodies have not been adequately characterized, which casts doubt on the results reported in many scientific papers. This problem is compounded by a lack of suitable control experiments in many studies. In this article we review the history of the 'antibody characterization crisis', and we document efforts and initiatives to address the problem, notably for antibodies that target human proteins. We also present recommendations for a range of stakeholders - researchers, universities, journals, antibody vendors and repositories, scientific societies and funders - to increase the reproducibility of studies that rely on antibodies.
Keywords: RRID; YCharOS; antibody characterization; antibody validation; cell biology; immunology; inflammation; none; renewables; reproducibility.
© 2024, Kahn et al.
Conflict of interest statement
RK, HV, CL, DH, NP, RM, AL, CS, TE, RT, JT, LF, FF, AB, SL No competing interests declared, AB Co-founder and serving as CEO of SciCrunch Inc, a company that works with publishers to improve the scientific literature; the terms of this agreement have been approved by the COI office at the University of California at San Diego, MR Employed by Addgene, a company that may be affected financially by the opinions reported in this article, HC Employed by Abcam, a for-profit company that sells antibodies and which may be affected financially by the opinions reported in this article
Figures
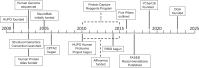
References
-
- Andersson S, Sundberg M, Pristovsek N, Ibrahim A, Jonsson P, Katona B, Clausson CM, Zieba A, Ramström M, Söderberg O, Williams C, Asplund A. Insufficient antibody validation challenges oestrogen receptor beta research. Nature Communications. 2017;8:15840. doi: 10.1038/ncomms15840. - DOI - PMC - PubMed
-
- Andrews NP, Boeckman JX, Manning CF, Nguyen JT, Bechtold H, Dumitras C, Gong B, Nguyen K, van der List D, Murray KD, Engebrecht J, Trimmer JS. A toolbox of IgG subclass-switched recombinant monoclonal antibodies for enhanced multiplex immunolabeling of brain. eLife. 2019;8:e43322. doi: 10.7554/eLife.43322. - DOI - PMC - PubMed
-
- Ayoubi R, Ryan J, Biddle MS, Alshafie W, Fotouhi M, Bolivar SG, Ruiz Moleon V, Eckmann P, Worrall D, McDowell I, Southern K, Reintsch W, Durcan TM, Brown C, Bandrowski A, Virk H, Edwards AM, McPherson P, Laflamme C. Scaling of an antibody validation procedure enables quantification of antibody performance in major research applications. eLife. 2023;12:RP91645. doi: 10.7554/eLife.91645. - DOI - PMC - PubMed

