The informatics of ADNI
- PMID: 39140398
- PMCID: PMC11485413
- DOI: 10.1002/alz.14099
The informatics of ADNI
Abstract
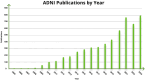
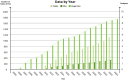
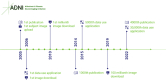
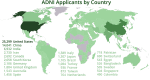
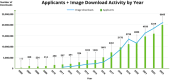
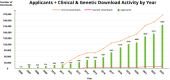
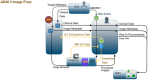
The Alzheimer's Disease Neuroimaging Initiative (ADNI) has revolutionized the landscape of Alzheimer's research through its Informatics Core, which has facilitated unprecedented data standardization and sharing. Over 20 years, ADNI established a robust informatics framework, enabling the validation of biomarkers and supporting global research efforts. The Informatics Core, centered at the Laboratory of Neuro Imaging (LONI), provides a comprehensive data hub that ensures data quality, accessibility, and security, fostering over 5600 publications and significant scientific advancements. By embracing open data sharing principles, ADNI set a gold standard in data transparency, allowing over 26,000 investigators from 169 countries to access and download a wealth of multimodal data. This collaborative approach not only accelerated biomarker discovery and drug development and advanced our understanding of Alzheimer's disease but also has served as a model for other research initiatives, demonstrating the transformative potential of carefully designed informatics models and shared data in driving global scientific progress. HIGHLIGHTS: Accelerating biomarker discovery and drug development for Alzheimer's disease. Alzheimer's Disease Neuroimaging Initiative's (ADNI's) open data sharing drives scientific progress. Data exploration and coupled analytics to data archives.
Keywords: Alzheimer's Disease Neuroimaging Initiative; Alzheimer's disease; biomarkers; data sharing; informatics; open data.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
None of the authors has any conflicts to disclose. The National Institutes of Health supported this effort. Author disclosures are available in the supporting information.
Figures
References
-
- GO FAIR . FAIR principles. Accessed March 29, 2024. https://www.go‐fair.org/fair‐principles/
-
- Springer Nature . Data repository guidance. Accessed April 18, 2024. https://www.nature.com/sdata/policies/repositories
-
- Wilhelm EE, Oster E, Shoulson I. Approaches and costs for sharing clinical research data. JAMA. 2014;311:1201‐1202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CAPMC/ CIHR/Canada
- Genentech, Inc.
- Araclon Biotech
- Janssen Alzheimer Immunotherapy Research & Development, LLC.
- Transition Therapeutics
- Cogstate
- EB/NIBIB NIH HHS/United States
- F. Hoffmann-La Roche Ltd
- Eli Lilly and Company
- Foundation for the National Institutes of Health
- Merck & Co., Inc.
- Eisai Inc.
- EuroImmun
- Biogen
- Johnson & Johnson Pharmaceutical Research & Development LLC.
- Alzheimer's Drug Discovery Foundation
- Servier
- Lumosity
- Bristol-Myers Squibb Company
- Piramal Imaging
- Takeda Pharmaceutical Company
- Novartis Pharmaceuticals Corporation
- Meso Scale Diagnostics, LLC.
- CereSpir, Inc.
- Northern California Institute for Research and Education
- BioClinica, Inc.
- U19 AG024904/AG/NIA NIH HHS/United States
- GE Healthcare
- AbbVie, Alzheimer's Association
- P30 AG066530/AG/NIA NIH HHS/United States
- Pfizer Inc.
- Elan Pharmaceuticals, Inc.
- IXICO Ltd.
- NeuroRx Research
- U19AG024904/NH/NIH HHS/United States
- Neurotrack Technologies
- Fujirebio
- Lundbeck
LinkOut - more resources
Full Text Sources
Medical

