Clinical characterization of common pathogenic variants of SOD1-ALS in Germany
- PMID: 39141064
- PMCID: PMC11446975
- DOI: 10.1007/s00415-024-12564-1
Clinical characterization of common pathogenic variants of SOD1-ALS in Germany
Erratum in
-
Correction: Clinical characterization of common pathogenic variants of SOD1-ALS in Germany.J Neurol. 2025 Mar 8;272(4):259. doi: 10.1007/s00415-025-12952-1. J Neurol. 2025. PMID: 40056187 Free PMC article. No abstract available.
Abstract
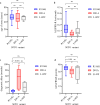
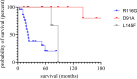
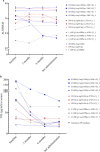
Pathogenic variants in the Cu/Zn superoxide dismutase (SOD1) gene can be detected in approximately 2% of sporadic and 11% of familial amyotrophic lateral sclerosis (ALS) patients in Europe. We analyzed the clinical phenotypes of 83 SOD1-ALS patients focusing on patients carrying the most frequent (likely) pathogenic variants (R116G, D91A, L145F) in Germany. Moreover, we describe the effect of tofersen treatment on ten patients carrying these variants. R116G patients showed the most aggressive course of disease with a median survival of 22.0 months compared to 198.0 months in D91A and 87.0 months in L145F patients (HR 7.71, 95% CI 2.89-20.58 vs. D91A; p < 0.001 and HR 4.25, 95% CI 1.55-11.67 vs. L145F; p = 0.02). Moreover, R116G patients had the fastest median ALSFRS-R progression rate with 0.12 (IQR 0.07-0.20) points lost per month. Median diagnostic delay was 10.0 months (IQR 5.5-11.5) and therefore shorter compared to 57.5 months (IQR 14.0-83.0) in D91A (p < 0.001) and 21.5 months (IQR 5.8-38.8) in L145F (p = 0.21) carriers. As opposed to D91A carriers (50.0%), 96.2% of R116G (p < 0.001) and 100.0% of L145F (p = 0.04) patients reported a positive family history. During tofersen treatment, all patients showed a reduction of neurofilament light chain (NfL) serum levels, independent of the SOD1 variant. Patients with SOD1-ALS carrying R116G, D91A, or L145F variants show commonalities, but also differences in their clinical phenotype, including a faster progression rate with shorter survival in R116G, and a comparatively benign disease course in D91A carriers.
Keywords: SOD1; Amyotrophic lateral sclerosis; Clinical phenotype; Motor neuron disease; Tofersen.
© 2024. The Author(s).
Conflict of interest statement
JD reports speaker honoraria from Biogen Inc.. SP received honoraria as a speaker/consultant from Biogen GmbH, Roche, Novartis, Teva, Cytokinetics Inc., Desitin, Italfarmaco, Zambon, Amylyx; and grants from DGM e.V, Federal Ministry of Education and Research, German Israeli Foundation for Scientific Research and Development, EU Joint Programme for Neurodegenerative Disease Research and Neurodegenerative Disease Research (NDR) outside the submitted work. TM is on the advisory board of Biogen and has received consulting fees from Biogen. TM is co-founder and shareholder of the Ambulanzpartner Soziotechnologie APST GmbH, which received a research grant from Biogen. PK reports participation on Advisory Boards of Biogen. HT reports grants or contracts from Sanofi Genzyme, German Multiple Sclerosis Society (DMSG), AMSEL Ursula-Späth-Stiftung, Bayern-DMSG, Deutsche MS-Stiftung, Ministry of Science, Research and Arts of the State Baden-Württemberg (MWK-BW), Chemische Fabrik Karl Bucher. HT received consulting fees from Merck, Novartis, Roche and received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Alexion, Bayer, Biogen, Celgene, GSK, Janssen, Merck, Novartis, Roche, Sanofi Genzyme, Siemens, TEVA, Viatris. HT reports support for attending meetings and/or travel from Janssen, Merck, Novartis, Roche, Sanofi Genzyme. He reports leadership or fiduciary role in DGLN, DMSG and AMSEL. ACL is a member of Advisory Boards of Roche Pharma AG, Biogen, Alector and Amylyx. He received compensation for talks from Biologix, the German Society of Neurology, Biogen, Springer Medicine, Amylyx and the company Streamed Up! GmbH. He is involved in trials which are sponsored by Amylyx, Ferrer International, Novartis Research and Development, Mitsubishi Tanabe, Apellis Pharmaceuticals, Alexion, Orion Pharma, the European Union, BMBF, Biogen and Orphazyme, Ionis Pharmaceuticals, QurAlis and Alector.
Figures

References
-
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX et al (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362:59–62. 10.1038/362059a0 - PubMed
-
- Chiò A, Calvo A, Moglia C, Mazzini L, Mora G, PARALS Study Group (2011) Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry 82:740–746. 10.1136/jnnp.2010.235952 - PubMed
-
- Rosenbohm A, Peter RS, Erhardt S, Lulé D, Rothenbacher D, Ludolph AC, Nagel G, ALS Registry Study Group (2017) Epidemiology of amyotrophic lateral sclerosis in Southern Germany. J Neurol 264:749–757. 10.1007/s00415-017-8413-3 - PubMed
-
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GY, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72:245–256. 10.1016/j.neuron.2011.09.011 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

