The real-world safety profile of tirzepatide: pharmacovigilance analysis of the FDA Adverse Event Reporting System (FAERS) database
- PMID: 39141075
- PMCID: PMC11473560
- DOI: 10.1007/s40618-024-02441-z
The real-world safety profile of tirzepatide: pharmacovigilance analysis of the FDA Adverse Event Reporting System (FAERS) database
Abstract
Purpose: Randomized controlled trials with tirzepatide (TZP) displayed unprecedented glucose and body weight lowering efficacy in individuals with type 2 diabetes and/or obesity and a safety profile similar to that of glucagon-like peptide-1 receptor agonists (GLP-1RA), mainly characterized by gastrointestinal (GI) adverse events (AE). Concerns on diabetic retinopathy, pancreato-biliary disorders, and medullary thyroid cancer were also addressed. We aimed to investigate whether the same safety issues emerged from the FDA Adverse Event Reporting System (FAERS) post-marketing surveillance database.
Methods: OpenVigil 2.1-MedDRA-v24 and AERSMine (data 2004Q1-2023Q3) were used to query the FAERS database. Reports of GI AE, diabetic retinopathy, pancreato-biliary disorders, and medullary thyroid cancer were investigated. The analysis was then filtered for age, gender, and designation as primary suspect. AE occurrence with TZP was compared to insulin, sodium-glucose cotransporter-2 inhibitors, metformin, and GLP-1RA.
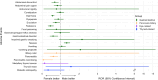
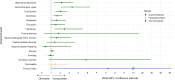
Results: Disproportionate reporting of GI [i.e., nausea (ROR 4.01, 95% CI 3.85-4.19)] and pancreato-biliary disorders [i.e., pancreatitis (ROR 3.63, 95% CI 3.15-4.19)], diabetic retinopathy (ROR 4.14, 95% CI 2.34-7.30), and medullary thyroid cancer (ROR 13.67, 95% CI 4.35-42.96) was detected. TZP exhibited a similar risk of GI AE and medullary thyroid cancer and a lower risk of most pancreato-biliary AE and diabetic retinopathy vs. GLP-1RA.
Conclusions: TZP was associated with an increased risk of specific AE. However, its safety profile was similar to that of GLP-1RA, without increased risk of pancreato-biliary AE, diabetic retinopathy, and medullary thyroid cancer.
Keywords: Adverse events; GIP; Tirzepatide; Type 2 diabetes.
© 2024. The Author(s).
Conflict of interest statement
AC: AstraZeneca, Eli Lilly, Novo Nordisk, Roche Diagnostics, Sanofi Aventis. FG: AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Lifescan, Medimmune, Merck Sharp & Dohme, Medtronic, Novo Nordisk, Roche Diabetes Care, Sanofi Aventis. GPS: Amgen, Amryt, Eli Lilly, Farmitalia, Novo Nordisk, Sanofi; IC: Eli Lilly, Novo Nordisk; LDG: Eli Lilly, Lusofarmaco, Medtronic, MOVI SpA, Novo Nordisk, Roche Diabetes Care, Sanofi Aventis. LL: Abbott, AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Merck Sharp & Dohme, Medtronic, Menarini, MOVI SpA, Mundipharma, Novo Nordisk, Roche Diabetes Care, Sanofi Aventis, Terumo. MC: AstraZeneca, Eli Lilly, Guidotti, Novo Nordisk; SDM: Ascensia Diabetes Care, Eli Lilly, MOVI SpA, Roche Diabetes Care.
Figures


References
-
- Caruso I, Di Gioia L, Di Molfetta S, Cignarelli A, Palmer SC, Natale P et al (2023) Glucometabolic outcomes of GLP-1 receptor agonist-based therapies in patients with type 2 diabetes: a systematic review and network meta-analysis. EClinicalMedicine 64:102181. 10.1016/j.eclinm.2023.102181 - PMC - PubMed
-
- Patel H, Khunti K, Rodbard HW, Bajaj HS, Bray R, Kindracki Z et al (2024) Gastrointestinal adverse events and weight reduction in people with type 2 diabetes treated with tirzepatide in the SURPASS clinical trials. Diabetes Obes Metab 26:473–481. 10.1111/dom.15333 - PubMed
-
- Bezin J, Gouverneur A, Pénichon M, Mathieu C, Garrel R, Hillaire-Buys D et al (2023) GLP-1 receptor agonists and the risk of thyroid cancer. Diabetes Care 46:384–390. 10.2337/dc22-1148 - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

