Baricitinib Withdrawal and Retreatment in Patients With Severe Alopecia Areata: The BRAVE-AA1 Randomized Clinical Trial
- PMID: 39141364
- PMCID: PMC11325239
- DOI: 10.1001/jamadermatol.2024.2734
Baricitinib Withdrawal and Retreatment in Patients With Severe Alopecia Areata: The BRAVE-AA1 Randomized Clinical Trial
Abstract
Importance: Baricitinib has demonstrated efficacy for treating severe alopecia areata in adults. There is currently limited information about the need for continuous therapy after achieving scalp hair regrowth.
Objective: To report results from the randomized withdrawal period of the BRAVE-AA1 trial.
Design, setting, and participants: BRAVE-AA1 was a randomized, placebo-controlled, phase 3 randomized clinical trial with a treatment withdrawal substudy that was conducted at 70 centers in 3 countries beginning in March 2019. It included 654 adults with severe alopecia areata (AA) (Severity of Alopecia Tool [SALT] score ≥50) who were randomized 3:2:2 to receive treatment with baricitinib, 4 mg; baricitinib, 2 mg; or placebo. Data were analyzed in August 2023.
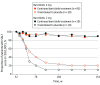
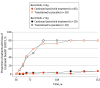
Intervention: At week 52, 154 patients who were responders (SALT score ≤20) were rerandomized 3:1 to continue to take their current dose of baricitinib or transition to placebo (randomized withdrawal). Responders randomized to placebo who experienced a loss of treatment benefit (>20-point worsening in SALT score) at any time after week 52 were retreated with their original baricitinib dose.
Main outcome and measures: The proportion of patients who lost treatment benefit through week 152 and the proportion of patients who recaptured response after retreatment. The last observation carried forward was used to impute missing or censored data.
Results: Of 654 patients who received treatment, the mean (SD) age was 37.1 (13.0) years, and there were 383 women (58.6%). At week 52, 10 of 39 responders taking baricitinib, 2 mg, and 30 of 115 responders taking baricitinib, 4 mg, were rerandomized to placebo. At 4 and 8 weeks of treatment withdrawal, 0% and 10% to 11% of patients, respectively, lost treatment benefit regardless of dose. At week 152, 80% of patients had lost benefit compared with 7% for those who continued baricitinib therapy for both dose groups. Within the follow-up observation periods, 5 of 8 patients taking 2 mg (63%) and 21 of 24 patients taking 4 mg (87.5%) recaptured a SALT score of 20 or less response after retreatment.
Conclusions and relevance: Severe AA is a chronic, relapsing condition, and this randomized clinical trial found that withdrawal of therapy for a patient population with severe AA who had achieved meaningful hair regrowth after 1 year of treatment with baricitinib resulted in loss of benefit for almost all patients, indicating that continued therapy is required to maintain hair regrowth.
Trial registration: ClinicalTrials.gov Identifier: NCT03570749.
Conflict of interest statement
Figures