Efficacy and safety of bintrafusp alfa in 2 phase I expansion cohorts with advanced HCC
- PMID: 39141577
- PMCID: PMC11643128
- DOI: 10.1097/HEP.0000000000001054
Efficacy and safety of bintrafusp alfa in 2 phase I expansion cohorts with advanced HCC
Abstract
Background and aims: Simultaneous inhibition of the TGF-β and programmed cell death 1 ligand 1 pathways provides a potential novel treatment approach. Bintrafusp alfa, a first-in-class bifunctional fusion protein composed of the extracellular domain of TGF-βRII (a TGF-β "trap") fused to a human IgG1 monoclonal antibody blocking programmed cell death 1 ligand 1, was evaluated in patients with advanced HCC.
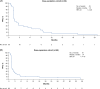
Approach and results: In this global, open-label, phase I study (NCT02517398), patients with programmed cell death 1 ligand 1-unselected HCC who failed or were intolerant to ≥1 line of sorafenib received bintrafusp alfa 1200 mg every 2 weeks in a dose-escalation (n = 38) or dose-expansion (n = 68) cohort until confirmed progression, unacceptable toxicity, or trial withdrawal. The primary endpoint was the best overall response per Response Evaluation Criteria in Solid Tumors version 1.1 by an independent review committee. Secondary endpoints included investigator-assessed best overall response, safety, and pharmacokinetics. Median follow-up times (range) were 41.4 (39.8-44.2) and 38.6 (33.5-39.7) months in the dose-escalation and dose-expansion cohorts, respectively. The objective response rate was below the prespecified 20% objective response rate threshold set to evaluate the efficacy of bintrafusp alfa in both cohorts (10.5% and 8.8%, respectively). Median overall survival and progression-free survival, respectively, were 13.8 and 1.5 months in the dose-escalation cohort and 13.5 and 1.4 months in the dose-expansion cohort. Treatment-related adverse events occurred in 78.9% and 64.7% of patients in the respective cohorts (grade ≥3 in 18.4% and 25.0% of patients).
Conclusions: Bintrafusp alfa showed moderate clinical activity and a safety profile consistent with previous studies of bintrafusp alfa in patients with advanced HCC.
Conflict of interest statement
Ho Yeong Lim consults, advises, and is on the speakers’ bureau for Bayer. He consults and advises AstraZeneca, Bayer, Eisai, the healthcare business of Merck KGaA, Darmstadt, Germany, and Roche. Jeong Heo is on the speakers’ bureau and received grants from Gilead, Roche, and Yuhan. He consults and advises AstraZeneca. Julio A. Peguero is employed by and owns stock in Oncology Consultants. He is on the speakers’ bureau for Agendia, Guardant Health, and Tempus. He received grants from AbbVie, Amgen, Astellas, Bavarian Nordic, BerGenBio, Boehringer Ingelheim, Calithera, Dizal, eFFECTOR Therapeutics, Eli Lilly, Epizyme, Genentech/Roche, Immatics, ImmunityBio, Immutep, IncMed, Incyte, Ipsen, Janssen, Kechow Pharma, Loxo/Lilly, Macrogenics, the healthcare business of Merck KGaA, Darmstadt, Germany, Mirati Therapeutics, Natera, Novartis, NovoCure, Sermonix, Tersera, Thrive Earlier Detection, and Turning Point. He owns stock in Roche, Spectrum, Tersera, and Zogen. Thomas Decaens advises and received grants from AbbVie, AstraZeneca, Bayer, Bristol Myers Squibb, Gilead, Ipsen, MSD, and Roche. He advises Becton Dickinson, BTG, Eisai, Guerbet, Sanofi, Sirtex, and Terumo. He received grants from ArQule, Enyopharma, Genoscience, and Guerbet. Fabrice Barlesi reports personal fees from AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Lilly Oncology, the healthcare business of Merck KGaA, Darmstadt, Germany Novartis, Pierre Fabre, Pfizer, Roche, and Takeda. Markus H. Moehler consults, advises, and received grants from Bristol Myers Squibb, Merck, and the healthcare business of Merck KGaA, Darmstadt, Germany. He consults and advises AstraZeneca. He is on the speakers’ bureau and received grants from the Falk Foundation. He consults and received grants from Novartis. He advises and is on the speakers’ bureau for Taiho. He is on the speakers’ bureau and received grants from Lilly. He advises Idience. He is on the speakers’ bureau for MCI Group, Roche, Sanofi, and Servier. He received grants from AIO, Amgen, the European Organisation for Research and Treatment of Cancer, and German Cancer Aid. Genevieve Jehl is employed by the healthcare business of Merck KGaA, Darmstadt, Germany S. Peter Eggleton is employed by Merck Serono Ltd., Feltham, UK, an affiliate of Merck KGaA, Darmstadt, Germany. Marcis Bajars is employed by the healthcare business of Merck KGaA, Darmstadt, Germany. James L. Gulley advises the healthcare business of Merck KGaA, Darmstadt, Germany. The remaining author has no conflicts to report.
Figures



References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, et al. . Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38:4317–4345. - PubMed
-
- Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, et al. . Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv238–iv255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

