High NEDA and No PIRA in Natalizumab-Treated Patients With Pediatric-Onset Multiple Sclerosis
- PMID: 39141876
- PMCID: PMC11379434
- DOI: 10.1212/NXI.0000000000200303
High NEDA and No PIRA in Natalizumab-Treated Patients With Pediatric-Onset Multiple Sclerosis
Abstract
Background and objectives: Although pediatric-onset multiple sclerosis (POMS) is characterized by a more rapid accumulation of CNS inflammation than adult-onset MS (AOMS), the therapeutic algorithms applied in POMS are usually based on AOMS therapeutic outcomes. To define a high-efficacy treatment (HET)-based strategy to treat POMS, we designed an observational retrospective study aimed at evaluating the efficacy and safety of natalizumab (NTZ) in naïve POMS and AOMS.
Methods: Starting from 160 patients, we applied a 2:1 (adult:pediatric) matching on propensity scores and obtained 32 patients with NTZ-treated POMS and 64 with AOMS, estimated from a multivariable logistic regression model. All patients were clinically and radiologically followed up every 6 months for a mean period of 46.0 ± 26.9 months.
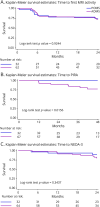
Results: Following re-baseline at month 6, no difference (log-rank test: p = 0.924) in new and enlarging T2 white matter lesions, postcontrast T1 lesions, and relapse rate were observed between POMS and AOMS throughout the study. Progression independent of relapse activity (PIRA) was never observed in POMS, while 9 of 64 patients with AOMS (12.5%) had PIRA events during the follow-up (40.0 ± 25.9 months; log-rank p value 0.0156). JCV seroconversion rate during NTZ infusion did not differ between POMS and AOMS (log-rank test p = 0.3231). Finally, no serious adverse event was observed in both POMS and AOMS.
Discussion: The favorable outcomes observed on clinical, especially in PIRA, and radiologic parameters strongly support the use of NTZ as a first-choice HET in POMS.
Conflict of interest statement
M. Puthenparampil report travel grants, consultancy, and board membership from Almirall, Teva, Sanofi Genzyme, Merck Serono, Biogen Italy, Novartis, Bristol Myers Squibb, Janssen, Sandoz, and Alexion. M. Ponzano, G. Zanotelli, A. Miscioscia, M. Nosadini, S. Sartori, F. Rinaldi have nothing to disclose. P. Perini reports grants from Almirall, Teva, Sanofi Genzyme, Merck Serono, Biogen Italy, Novartis, Roche, Alexion, Janssen, Bristol Mayer Squibb; consultancy for Novartis, Biogen Italy, Sanofi Genzyme, Roche, Janssen, Bristol Mayer Squibb. RF report grants from Almirall, Teva, Sanofi Genzyme, Merck Serono, Biogen Italy, Novartis, consultancy for Novartis, Biogen Italy, Sanofi Genzyme. P. Gallo reports grant, consultancy, and board membership for Almirall, Teva, Sanofi Genzyme, Merck Serono, Biogen Italy, Novartis, Roche, Bristol Myers Squibb, Janssen, and Alexion. Go to
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
