Regulator of G-protein signaling expression in human intestinal enteroendocrine cells and potential role in satiety hormone secretion in health and obesity
- PMID: 39142076
- PMCID: PMC11367526
- DOI: 10.1016/j.ebiom.2024.105283
Regulator of G-protein signaling expression in human intestinal enteroendocrine cells and potential role in satiety hormone secretion in health and obesity
Abstract
Background: Gut L-type enteroendocrine cells (EECs) are intestinal chemosensory cells that secrete satiety hormones GLP-1 and PYY in response to activation of G-protein coupled receptors (GPCRs) by luminal components of nutrient digestion and microbial fermentation. Regulator of G-protein Signaling (RGS) proteins are negative regulators of GPCR signaling. The expression profile of RGS in EECs, and their potential role in satiety hormone secretion and obesity is unknown.
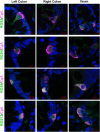
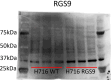
Methods: Transcriptomic profiling of RGS was completed in native colonic EECs was completed using single-cell RNA sequencing (scRNA-Seq) in lean and obesity, and human jejunal EECs with data obtained from a publicly available RNAseq dataset (GSE114853). RGS validation studies were completed using whole mucosal intestinal tissue obtained during endoscopy in 61 patients (n = 42 OB, n = 19 Lean); a subset of patients' postprandial plasma was assayed for GLP-1 and PYY. Ex vivo human intestinal cultures and in vitro NCI-H716 cells overexpressing RGS9 were exposed to GLP-1 secretagogues in conjunction with a nonselective RGS-inhibitor and assayed for GLP-1 secretion.
Findings: Transcriptomic profiling of colonic and jejunal enteroendocrine cells revealed a unique RGS expression profile in EECs, and further within GLP-1+ L-type EECs. In obesity the RGS expression profile was altered in colonic EECs. Human gut RGS9 expression correlated positively with BMI and negatively with postprandial GLP-1 and PYY. RGS inhibition in human intestinal cultures increased GLP-1 release from EECs ex vivo. NCI-H716 cells overexpressing RGS9 displayed defective nutrient-stimulated GLP-1 secretion.
Interpretation: This study introduces the expression profile of RGS in human EECs, alterations in obesity, and suggests a role for RGS proteins as modulators of GLP-1 and PYY secretion from intestinal EECs.
Funding: AA is supported by the NIH(C-Sig P30DK84567, K23 DK114460), a Pilot Award from the Mayo Clinic Center for Biomedical Discovery, and a Translational Product Development Fund from The Mayo Clinic Center for Clinical and Translational Science Office of Translational Practice in partnership with the University of Minnesota Clinical and Translational Science Institute.
Keywords: Enteroendocrine cells; GLP-1; L-cells; Obesity; PYY; RGS; scRNA-Seq.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests AA is a stockholder in Gila Therapeutics, Phenomix Sciences. AA provides consulting services for Rhythm Pharmaceuticals, General Mills, Amgen, RareStone, and Bausch Health. DH provides consulting services for Novo Nordisk and receives presentation compensation from the Obesity Medicine Association and The Menopause Society.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

