Loss of NAMPT and SIRT2 but not SIRT1 attenuate GLO1 expression and activity in human skeletal muscle
- PMID: 39142179
- PMCID: PMC11367650
- DOI: 10.1016/j.redox.2024.103300
Loss of NAMPT and SIRT2 but not SIRT1 attenuate GLO1 expression and activity in human skeletal muscle
Abstract
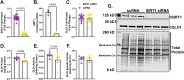
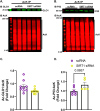
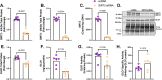
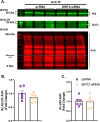
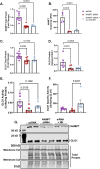
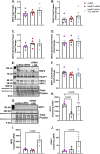
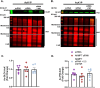
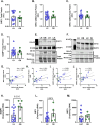
Glyoxalase I (GLO1) is the primary enzyme for detoxification of the reactive dicarbonyl methylglyoxal (MG). Loss of GLO1 promotes accumulation of MG resulting in a recapitulation of diabetic phenotypes. We previously demonstrated attenuated GLO1 protein in skeletal muscle from individuals with type 2 diabetes (T2D). However, whether GLO1 attenuation occurs prior to T2D and the mechanisms regulating GLO1 abundance in skeletal muscle are unknown. GLO1 expression and activity were determined in skeletal muscle tissue biopsies from 15 lean healthy individuals (LH, BMI: 22.4 ± 0.7) and 5 individuals with obesity (OB, BMI: 32.4 ± 1.3). GLO1 protein was attenuated by 26 ± 0.3 % in OB compared to LH skeletal muscle (p = 0.019). Similar reductions for GLO1 activity were observed (p = 0.102). NRF2 and Keap1 expression were equivocal between groups despite a 2-fold elevation in GLO1 transcripts in OB skeletal muscle (p = 0.008). GLO1 knock-down (KD) in human immortalized myotubes promoted downregulation of muscle contraction and organization proteins indicating the importance of GLO1 expression for skeletal muscle function. SIRT1 KD had no effect on GLO1 protein or activity whereas, SIRT2 KD attenuated GLO1 protein by 28 ± 0.29 % (p < 0.0001) and GLO1 activity by 42 ± 0.12 % (p = 0.0150). KD of NAMPT also resulted in attenuation of GLO1 protein (28 ± 0.069 %, p = 0.003), activity (67 ± 0.09 %, p = 0.011) and transcripts (50 ± 0.13 %, p = 0.049). Neither the provision of the NAD+ precursors NR nor NMN were able to prevent this attenuation in GLO1 protein. However, NR did augment GLO1 specific activity (p = 0.022 vs NAMPT KD). These perturbations did not alter GLO1 acetylation status. SIRT1, SIRT2 and NAMPT protein levels were all equivocal in skeletal muscle tissue biopsies from individuals with obesity and lean individuals. These data implicate NAD+-dependent regulation of GLO1 in skeletal muscle independent of altered GLO1 acetylation and provide rationale for exploring NR supplementation to rescue attenuated GLO1 abundance and activity in conditions such as obesity.
Keywords: Dicarbonyl stress; Methylglyoxal; NAD(+); Nicotinamide riboside.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Miranda E.R., Haus J.M. Glyoxalase I is a novel target for the prevention of metabolic derangement. Pharmacol. Therapeut. 2023;250 - PubMed
-
- Zhao Y., Wang P., Sang S. Dietary genistein inhibits methylglyoxal-induced advanced glycation end product formation in mice fed a high-fat diet. J. Nutr. 2019;149:776–787. - PubMed
-
- Maessen D.E., et al. Delayed intervention with pyridoxamine improves metabolic function and prevents adipose tissue inflammation and insulin resistance in high-fat diet–induced obese mice. Diabetes. 2016;65:956–966. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

