Causal evidence of a line attractor encoding an affective state
- PMID: 39142337
- PMCID: PMC11499281
- DOI: 10.1038/s41586-024-07915-x
Causal evidence of a line attractor encoding an affective state
Abstract
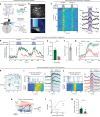
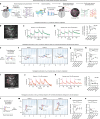
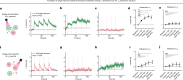
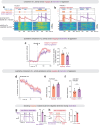
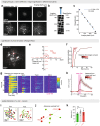
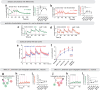
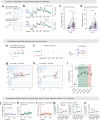
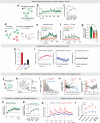
Continuous attractors are an emergent property of neural population dynamics that have been hypothesized to encode continuous variables such as head direction and eye position1-4. In mammals, direct evidence of neural implementation of a continuous attractor has been hindered by the challenge of targeting perturbations to specific neurons within contributing ensembles2,3. Dynamical systems modelling has revealed that neurons in the hypothalamus exhibit approximate line-attractor dynamics in male mice during aggressive encounters5. We have previously hypothesized that these dynamics may encode the variable intensity and persistence of an aggressive internal state. Here we report that these neurons also showed line-attractor dynamics in head-fixed mice observing aggression6. This allowed us to identify and manipulate line-attractor-contributing neurons using two-photon calcium imaging and holographic optogenetic perturbations. On-manifold perturbations yielded integration of optogenetic stimulation pulses and persistent activity that drove the system along the line attractor, while transient off-manifold perturbations were followed by rapid relaxation back into the attractor. Furthermore, single-cell stimulation and imaging revealed selective functional connectivity among attractor-contributing neurons. Notably, individual differences among mice in line-attractor stability were correlated with the degree of functional connectivity among attractor-contributing neurons. Mechanistic recurrent neural network modelling indicated that dense subnetwork connectivity and slow neurotransmission7 best recapitulate our empirical findings. Our work bridges circuit and manifold levels3, providing causal evidence of continuous attractor dynamics encoding an affective internal state in the mammalian hypothalamus.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

