Deficiency in the mitophagy mediator Parkin accelerates murine skin allograft rejection
- PMID: 39142471
- PMCID: PMC11588513
- DOI: 10.1016/j.ajt.2024.08.005
Deficiency in the mitophagy mediator Parkin accelerates murine skin allograft rejection
Abstract
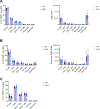
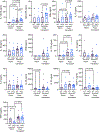
Alterations in mitochondrial function and associated quality control programs, including mitochondrial-specific autophagy, termed mitophagy, are gaining increasing recognition in the context of disease. However, the role of mitophagy in organ transplant rejection remains poorly understood. Using mice deficient in Parkin, a ubiquitin ligase that tags damaged or dysfunctional mitochondria for autophagic clearance, we assessed the impact of Parkin-dependent mitophagy on skin-graft rejection. We observed accelerated graft loss in Parkin-deficient mice across multiple skin graft models. Immune cell distributions posttransplant were largely unperturbed compared to wild-type; however, the CD8+ T cells of Parkin-deficient mice expressed more T-bet, IFNγ, and Ki67, indicating greater priming toward effector function. This was accompanied by increased circulating levels of IL-12p70 in Parkin-deficient mice. Using a mixed leukocyte reaction, we demonstrated that naïve Parkin-deficient CD4+ and CD8+ T cells exhibit enhanced activation marker expression and proliferative responses to alloantigen, which were attenuated with administration of a pharmacological mitophagy inducer (p62-mediated mitophagy inducer), known to increase mitophagy in the absence of a functional PINK1-Parkin pathway. These findings indicate a role for Parkin-dependent mitophagy in curtailing skin-graft rejection.
Keywords: Parkin; T cells; mitochondria; mitophagy; transplant.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Figures




References
-
- Hall BM, Gurley KE, Dorsch SE. The possible role of cytotoxic T cells in the mediation of first-set allograft rejection. Transplantation. Sep 1985;40(3):336–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

