Caspase-11 mediated inflammasome activation in macrophages by systemic infection of A. actinomycetemcomitans exacerbates arthritis
- PMID: 39143049
- PMCID: PMC11324795
- DOI: 10.1038/s41368-024-00315-x
Caspase-11 mediated inflammasome activation in macrophages by systemic infection of A. actinomycetemcomitans exacerbates arthritis
Abstract
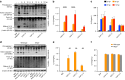
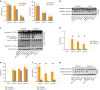
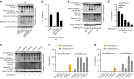
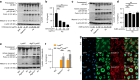
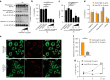
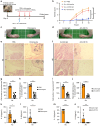
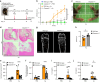
Clinical studies have shown that Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans) is associated with aggressive periodontitis and can potentially trigger or exacerbate rheumatoid arthritis (RA). However, the mechanism is poorly understood. Here, we show that systemic infection with A. actinomycetemcomitans triggers the progression of arthritis in mice anti-collagen antibody-induced arthritis (CAIA) model following IL-1β secretion and cell infiltration in paws in a manner that is dependent on caspase-11-mediated inflammasome activation in macrophages. The administration of polymyxin B (PMB), chloroquine, and anti-CD11b antibody suppressed inflammasome activation in macrophages and arthritis in mice, suggesting that the recognition of lipopolysaccharide (LPS) in the cytosol after bacterial degradation by lysosomes and invasion via CD11b are needed to trigger arthritis following inflammasome activation in macrophages. These data reveal that the inhibition of caspase-11-mediated inflammasome activation potentiates aggravation of RA induced by infection with A. actinomycetemcomitans. This work highlights how RA can be progressed by inflammasome activation as a result of periodontitis-associated bacterial infection and discusses the mechanism of inflammasome activation in response to infection with A. actinomycetemcomitans.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Socransky, S. S., Haffajee, A. D., Cugini, M. A., Smith, C. & Kent, R. L. Microbial complexes in subgingival plaque. J. Clin. Periodontol.25, 134–144 (1998). - PubMed
-
- Ding, C., Ji, X., Chen, X., Xu, Y. & Zhong, L. TNF-α gene promoter polymorphisms contribute to periodontitis susceptibility: Evidence from 46 studies. J. Clin. Periodontol.41, 748–759 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 21J00572/MEXT | Japan Society for the Promotion of Science (JSPS)
- 16H05186/MEXT | Japan Society for the Promotion of Science (JSPS)
- 15H04730/MEXT | Japan Society for the Promotion of Science (JSPS)
- 16K08772/MEXT | Japan Society for the Promotion of Science (JSPS)
- 17K12004/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

