Dose dense versus 3 weekly AC during neoadjuvant chemoimmunotherapy for triple negative breast cancer
- PMID: 39143082
- PMCID: PMC11324725
- DOI: 10.1038/s41523-024-00676-w
Dose dense versus 3 weekly AC during neoadjuvant chemoimmunotherapy for triple negative breast cancer
Abstract
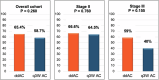
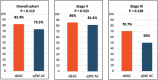
Neoadjuvant pembrolizumab plus chemotherapy (P + CT) has emerged as a standard of care for stage II-III triple-negative breast cancer (TNBC). However, the best anthracycline-cyclophosphamide (AC) schedule remains to be determined. While the KEYNOTE-522 regimen employs AC every 3 weeks (q3w AC), previous studies have shown overall survival benefits of dose-dense regimens for early-stage breast cancer. The Neo-Real study (GBECAM-0123) is a real-world data effort evaluating patients with TNBC treated with neoadjuvant P + CT in ten cancer centers since July 2020. The objective of this analysis was to evaluate the effectiveness and safety of dose-dense AC (ddAC) versus q3w AC. Among 333 patients included until November 2023, 311 completed neoadjuvant therapy and 279 underwent surgery with pathology reports available; ddAC was used in 58.2% and q3w AC in 41.8% of the cases. Most patients (69.1%) had stage II TNBC. A pCR was observed in 65.4% with ddAC and 58.7% with q3w AC (P = 0.260), while RCB 0-1 occurred in 82.4% and 73.5%, respectively (P = 0.115). Patients with stage III disease had a numerically higher pCR with ddAC (59% vs 40%, P = 0.155), while pCR rates were similar regardless of AC regimen in stage II disease (66.6% vs 64.5%; P = 0.760). While no significant disparities in drug discontinuation was noted, ddAC showed a trend towards higher rates of grade ≥3 AE (40.5% vs. 30.7%, P = 0.092). The Neo-Real study could not rule out a difference between ddAC and q3w AC during neoadjuvant P + CT. The observation of a potentially higher pCR with ddAC in stage III disease warrants further investigation.
© 2024. The Author(s).
Conflict of interest statement
The Authors declare no Competing Non-Financial Interests but the following Competing Financial Interests: R.C.B.: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo, Nestle Health Science, Addium, Gilead, MSD, BMS, AstraZeneca, Ache, Pfizer. Financial support for educational programs and symposia: AstraZeneca, Daiichi-Sankyo, MSD. Institutional Research grant: Novartis, AstraZeneca. J.B.: Speaker fees and/or honoraria for consulting or advisory functions: AstraZeneca, Daiichi-Sankyo, Lilly, Gilead, Pfizer, Novartis, MSD, Roche, Knight Pharmaceuticals. Financial support for educational programs and symposia: Roche, Daiichi-Sankyo. D.D.R.: Speaker fees and/or honoraria for consulting or advisory functions: AstraZeneca, Daiichi-Sankyo, Lilly, Libbs, Pfizer, Novartis, Roche, GSK, Sanofi, Amgen, Zodiac Pharma. Financial support for educational programs and symposia: Roche. D.A.S.: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo. Financial support for educational programs and symposia: AstraZeneca. J.A.P.A.: Speaker fees and/or honoraria for consulting or advisory functions: Novartis, AstraZeneca, MSD, Lilly. D.M.G.: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo, Teva, Roche, AstraZeneca, Pfizer, Lilly, Novartis. Financial support for educational programs and symposia: AstraZeneca, Libbs, Roche. Research grant: Novartis. B.M.Z.: AstraZeneca, Daiichi-Sankyo, Eli Lilly, Gilead, Pfizer, Novartis, MSD, Roche, Addium. A.F.: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo, Novartis, Gilead, MSD, BMS, AstraZeneca, Pfizer. Financial support for educational programs and symposia: AstraZeneca, Daiichi-Sankyo, MSD, Novartis. C.H.A.: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo, Gilead AstraZeneca, Novartis, MSD. Financial support for educational programs and symposia: AstraZeneca, Daiichi-Sankyo, MSD, Lilly, Rcohe, Novartis, Gilead, Medscape. R.C.: Speaker fees and/or honoraria for consulting or advisory functions: AstraZeneca, Daichii-Sankyo, Eli Lilly, Gilead, MSD, and GSK. M.M.F.M.: Speaker fees and/or honoraria for consulting or advisory functions: AstraZeneca, Daiichi-Sankyo, Eli Lilly, Gilead, Adium, Novartis, MSD, and Roche. Financial support for educational programs and symposia: AstraZeneca, Daiichi-Sankyo, Gilead, Eli Lilly, Roche, MSD and Novartis. P.M.H.: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo. L.T.: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo, MSD, AstraZeneca, Pfizer, Lilly, Novartis. Financial support for educational programs and symposia: AstraZeneca, Roche, Gilead. Institutional Research grant: Novartis. R.B.S.: Speaker fees and/or honoraria for consulting or advisory functions: AstraZeneca, Daiichi-Sankyo, Eli Lilly, Gilead, Libbs, Pfizer, Novartis, MSD, and Roche. Financial support for educational programs and symposia: AstraZeneca, Daiichi-Sankyo, Gilead, Eli Lilly, and MSD. Institutional Research grant: AstraZeneca, Daiichi-Sankyo. I.M.S., F.C.B., A.C.M.C., M.C.T., F.M., R.P.F., C.L.S., and Z.S.S. declare no conflict of interest.
Figures
References
-
- Schmid, P. et al. Neoadjuvant pembrolizumab or placebo plus chemotherapy followed by adjuvant pembrolizumab or placebo for early-stage triple-negative breast cancer: updated event-free survival results from the phase 3 KEYNOTE-522 study. Presented at the 2023 San Antonio Breast Cancer Symposium; December 5-9, 2023; San Antonio; 2023. p. abstract LBO1-01.
-
- Mittendorf, E. et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet (Lond., Engl.)396, 1090–1100 (2020). 10.1016/S0140-6736(20)31953-X - DOI - PubMed
LinkOut - more resources
Full Text Sources

