Lamin A/C deficiency-mediated ROS elevation contributes to pathogenic phenotypes of dilated cardiomyopathy in iPSC model
- PMID: 39143095
- PMCID: PMC11324749
- DOI: 10.1038/s41467-024-51318-5
Lamin A/C deficiency-mediated ROS elevation contributes to pathogenic phenotypes of dilated cardiomyopathy in iPSC model
Abstract
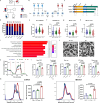
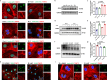
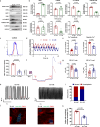
Mutations in the nuclear envelope (NE) protein lamin A/C (encoded by LMNA), cause a severe form of dilated cardiomyopathy (DCM) with early-onset life-threatening arrhythmias. However, molecular mechanisms underlying increased arrhythmogenesis in LMNA-related DCM (LMNA-DCM) remain largely unknown. Here we show that a frameshift mutation in LMNA causes abnormal Ca2+ handling, arrhythmias and disformed NE in LMNA-DCM patient-specific iPSC-derived cardiomyocytes (iPSC-CMs). Mechanistically, lamin A interacts with sirtuin 1 (SIRT1) where mutant lamin A/C accelerates degradation of SIRT1, leading to mitochondrial dysfunction and oxidative stress. Elevated reactive oxygen species (ROS) then activates the Ca2+/calmodulin-dependent protein kinase II (CaMKII)-ryanodine receptor 2 (RYR2) pathway and aggravates the accumulation of SUN1 in mutant iPSC-CMs, contributing to arrhythmias and NE deformation, respectively. Taken together, the lamin A/C deficiency-mediated ROS disorder is revealed as central to LMNA-DCM development. Manipulation of impaired SIRT1 activity and excessive oxidative stress is a potential future therapeutic strategy for LMNA-DCM.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
- 81922006/National Natural Science Foundation of China (National Science Foundation of China)
- 81870175/National Natural Science Foundation of China (National Science Foundation of China)
- 82370354/National Natural Science Foundation of China (National Science Foundation of China)
- 82370313/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Miscellaneous

