Crimean Congo hemorrhagic fever virus nucleoprotein and GP38 subunit vaccine combination prevents morbidity in mice
- PMID: 39143104
- PMCID: PMC11324950
- DOI: 10.1038/s41541-024-00931-y
Crimean Congo hemorrhagic fever virus nucleoprotein and GP38 subunit vaccine combination prevents morbidity in mice
Abstract
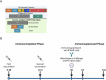
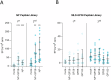
Immunizing mice with Crimean-Congo hemorrhagic fever virus (CCHFV) nucleoprotein (NP), glycoprotein precursor (GPC), or with the GP38 domain of GPC, can be protective when the proteins are delivered with viral vectors or as a DNA or RNA vaccine. Subunit vaccines are a safe and cost-effective alternative to some vaccine platforms, but Gc and Gn glycoprotein subunit vaccines for CCHFV fail to protect despite eliciting high levels of neutralizing antibodies. Here, we investigated humoral and cellular immune responses and the protective efficacy of recombinant NP, GP38, and GP38 forms (GP85 and GP160) associated with the highly glycosylated mucin-like (MLD) domain, as well as the NP + GP38 combination. Vaccination with GP160, GP85, or GP38 did not confer protection, and vaccination with the MLD-associated GP38 forms blunted the humoral immune responses to GP38, worsened clinical chemistry, and increased viral RNA in the blood compared to the GP38 vaccination. In contrast, NP vaccination conferred 100% protection from lethal outcome and was associated with mild clinical disease, while the NP + GP38 combination conferred even more robust protection by reducing morbidity compared to mice receiving NP alone. Thus, recombinant CCHFV NP alone is a promising vaccine candidate conferring 100% survival against heterologous challenge. Moreover, incorporation of GP38 should be considered as it further enhances subunit vaccine efficacy by reducing morbidity in surviving animals.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Nucleoside-Modified mRNA Vaccines Protect IFNAR-/- Mice against Crimean-Congo Hemorrhagic Fever Virus Infection.J Virol. 2022 Feb 9;96(3):e0156821. doi: 10.1128/JVI.01568-21. Epub 2021 Nov 24. J Virol. 2022. PMID: 34817199 Free PMC article.
-
The interplays between Crimean-Congo hemorrhagic fever virus (CCHFV) M segment-encoded accessory proteins and structural proteins promote virus assembly and infectivity.PLoS Pathog. 2020 Sep 21;16(9):e1008850. doi: 10.1371/journal.ppat.1008850. eCollection 2020 Sep. PLoS Pathog. 2020. PMID: 32956404 Free PMC article.
-
Immunization with DNA Plasmids Coding for Crimean-Congo Hemorrhagic Fever Virus Capsid and Envelope Proteins and/or Virus-Like Particles Induces Protection and Survival in Challenged Mice.J Virol. 2017 Apr 28;91(10):e02076-16. doi: 10.1128/JVI.02076-16. Print 2017 May 15. J Virol. 2017. PMID: 28250124 Free PMC article.
-
Structure and Characterization of Crimean-Congo Hemorrhagic Fever Virus GP38.J Virol. 2020 Mar 31;94(8):e02005-19. doi: 10.1128/JVI.02005-19. Print 2020 Mar 31. J Virol. 2020. PMID: 31996434 Free PMC article.
-
GP38 as a vaccine target for Crimean-Congo hemorrhagic fever virus.NPJ Vaccines. 2023 May 20;8(1):73. doi: 10.1038/s41541-023-00663-5. NPJ Vaccines. 2023. PMID: 37210392 Free PMC article.
Cited by
-
Engineering and structures of Crimean-Congo hemorrhagic fever virus glycoprotein complexes.Cell. 2025 Jan 23;188(2):303-315.e13. doi: 10.1016/j.cell.2024.11.008. Epub 2024 Dec 18. Cell. 2025. PMID: 39701101
-
Rapid, sensitive, and species-independent detection of Crimean Congo hemorrhagic fever virus nucleoprotein and GP38 antibodies.EBioMedicine. 2025 Jul 23;118:105857. doi: 10.1016/j.ebiom.2025.105857. Online ahead of print. EBioMedicine. 2025. PMID: 40706445 Free PMC article.
-
Single dose VSV-based vaccine protects mice against lethal heterologous Crimean-Congo hemorrhagic fever virus challenge.NPJ Vaccines. 2025 May 30;10(1):109. doi: 10.1038/s41541-025-01164-3. NPJ Vaccines. 2025. PMID: 40447644 Free PMC article.
-
Advancements and Challenges in Addressing Zoonotic Viral Infections with Epidemic and Pandemic Threats.Viruses. 2025 Feb 28;17(3):352. doi: 10.3390/v17030352. Viruses. 2025. PMID: 40143281 Free PMC article. Review.
References
-
- Elaldi, N. & Kaya, S. Crimean-Congo hemorrhagic fever. Jmid4, (2014).
-
- World Health Organization. Crimean-Congo haemorrhagic fever. https://www.who.int/news-room/fact-sheets/detail/crimean-congo-haemorrha... (2022).
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

