Origin and evolution of the bread wheat D genome
- PMID: 39143210
- PMCID: PMC11424481
- DOI: 10.1038/s41586-024-07808-z
Origin and evolution of the bread wheat D genome
Abstract
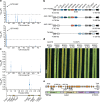
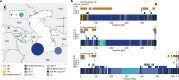
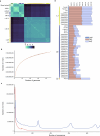
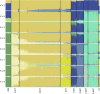
Bread wheat (Triticum aestivum) is a globally dominant crop and major source of calories and proteins for the human diet. Compared with its wild ancestors, modern bread wheat shows lower genetic diversity, caused by polyploidisation, domestication and breeding bottlenecks1,2. Wild wheat relatives represent genetic reservoirs, and harbour diversity and beneficial alleles that have not been incorporated into bread wheat. Here we establish and analyse extensive genome resources for Tausch's goatgrass (Aegilops tauschii), the donor of the bread wheat D genome. Our analysis of 46 Ae. tauschii genomes enabled us to clone a disease resistance gene and perform haplotype analysis across a complex disease resistance locus, allowing us to discern alleles from paralogous gene copies. We also reveal the complex genetic composition and history of the bread wheat D genome, which involves contributions from genetically and geographically discrete Ae. tauschii subpopulations. Together, our results reveal the complex history of the bread wheat D genome and demonstrate the potential of wild relatives in crop improvement.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Tadesse, W. et al. Genetic gains in wheat breeding and its role in feeding the world. Crop Breed. Genet. Genom.1, e190005 (2019).
MeSH terms
LinkOut - more resources
Full Text Sources

