Recognition and control of neutrophil extracellular trap formation by MICL
- PMID: 39143217
- PMCID: PMC11390483
- DOI: 10.1038/s41586-024-07820-3
Recognition and control of neutrophil extracellular trap formation by MICL
Abstract
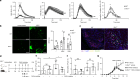
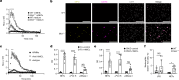
Regulation of neutrophil activation is critical for disease control. Neutrophil extracellular traps (NETs), which are web-like structures composed of DNA and neutrophil-derived proteins, are formed following pro-inflammatory signals; however, if this process is uncontrolled, NETs contribute to disease pathogenesis, exacerbating inflammation and host tissue damage1,2. Here we show that myeloid inhibitory C-type lectin-like (MICL), an inhibitory C-type lectin receptor, directly recognizes DNA in NETs; this interaction is vital to regulate neutrophil activation. Loss or inhibition of MICL functionality leads to uncontrolled NET formation through the ROS-PAD4 pathway and the development of an auto-inflammatory feedback loop. We show that in the context of rheumatoid arthritis, such dysregulation leads to exacerbated pathology in both mouse models and in human patients, where autoantibodies to MICL inhibit key functions of this receptor. Of note, we also detect similarly inhibitory anti-MICL autoantibodies in patients with other diseases linked to aberrant NET formation, including lupus and severe COVID-19. By contrast, dysregulation of NET release is protective during systemic infection with the fungal pathogen Aspergillus fumigatus. Together, we show that the recognition of NETs by MICL represents a fundamental autoregulatory pathway that controls neutrophil activity and NET formation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures











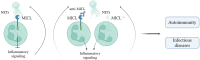
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

