Maternal serum PlGF associates with 3D power doppler ultrasound markers of utero-placental vascular development in the first trimester: the rotterdam periconception cohort
- PMID: 39143350
- PMCID: PMC11564232
- DOI: 10.1007/s10456-024-09939-3
Maternal serum PlGF associates with 3D power doppler ultrasound markers of utero-placental vascular development in the first trimester: the rotterdam periconception cohort
Abstract
Objective (s): Circulating angiogenic factors are used for prediction of placenta-related complications, but their associations with first-trimester placental development is unknown. This study investigates associations between maternal angiogenic factors and utero-placental vascular volume (uPVV) and utero-placental vascular skeleton (uPVS) as novel imaging markers of volumetric and morphologic (branching) development of the first-trimester utero-placental vasculature.
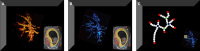
Methods: In 185 ongoing pregnancies from the VIRTUAL Placenta study, a subcohort of the ongoing prospective Rotterdam Periconception cohort, three-dimensional power Doppler ultrasounds of the placenta were obtained at 7-9-11 weeks gestational age (GA). The uPVV was measured as a parameter of volumetric development and reported the vascular quantity in cm3. The uPVS was generated as a parameter of morphologic (branching) development and reported the number of end-, bifurcation- crossing- or vessel points and total vascular length. At 11 weeks GA, maternal serum biomarkers suggested to reflect placental (vascular) development were assessed: placental growth factor (PlGF), soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng). sFlt-1/PlGF and sEng/PlGF ratios were calculated. Multivariable linear regression with adjustments was used to estimate associations between serum biomarkers and uPVV and uPVS trajectories.
Results: Serum PlGF was positively associated with uPVV and uPVS development (uPVV: β = 0.39, 95% CI = 0.15;0.64; bifurcation points: β = 4.64, 95% CI = 0.04;9.25; crossing points: β = 4.01, 95% CI = 0.65;7.37; total vascular length: β = 13.33, 95% CI = 3.09;23.58, all p-values < 0.05). sEng/PlGF ratio was negatively associated with uPVV and uPVS development. We observed no associations between sFlt-1, sEng or sFlt-1/PlGF ratio and uPVV and uPVS development.
Conclusion(s): Higher first-trimester maternal serum PlGF concentration is associated with increased first-trimester utero-placental vascular development as reflected by uPVV and uPVS. Clinical trial registration number Dutch Trial Register NTR6854.
Keywords: Angiogenesis; Angiogenic factors; Placenta; Preeclampsia; Spiral artery remodeling; sFlt-1.
© 2024. The Author(s).
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

