Rapid discovery of monoclonal antibodies by microfluidics-enabled FACS of single pathogen-specific antibody-secreting cells
- PMID: 39143416
- PMCID: PMC12167710
- DOI: 10.1038/s41587-024-02346-5
Rapid discovery of monoclonal antibodies by microfluidics-enabled FACS of single pathogen-specific antibody-secreting cells
Abstract
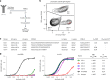
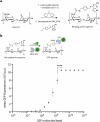
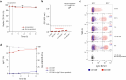
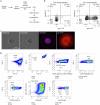
Monoclonal antibodies are increasingly used to prevent and treat viral infections and are pivotal in pandemic response efforts. Antibody-secreting cells (ASCs; plasma cells and plasmablasts) are an excellent source of high-affinity antibodies with therapeutic potential. Current methods to study antigen-specific ASCs either have low throughput, require expensive and labor-intensive screening or are technically demanding and therefore not widely accessible. Here we present a straightforward technology for the rapid discovery of monoclonal antibodies from ASCs. Our approach combines microfluidic encapsulation of single cells into an antibody capture hydrogel with antigen bait sorting by conventional flow cytometry. With our technology, we screened millions of mouse and human ASCs and obtained monoclonal antibodies against severe acute respiratory syndrome coronavirus 2 with high affinity (<1 pM) and neutralizing capacity (<100 ng ml-1) in 2 weeks with a high hit rate (>85% of characterized antibodies bound the target). By facilitating access to the underexplored ASC compartment, the approach enables efficient antibody discovery and immunological studies into the generation of protective antibodies.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: C.M.D. discloses membership of the Scientific Advisory Board of Fusion Antibodies and AI proteins. All other authors declare no competing interests.
Figures









References
MeSH terms
Substances
Grants and funding
- Studentship/Gates Cambridge Trust
- MSCA-IF-750772/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 Marie Skłodowska-Curie Actions (H2020 Excellent Science - Marie Skłodowska-Curie Actions)
- 900239/CUH | Addenbrooke's Charitable Trust, Cambridge University Hospitals (Addenbrooke's Charitable Trust, Cambridge University Hospitals NHS Foundation Trust)
- MC_UU_00025/12/RCUK | Medical Research Council (MRC)
- WT108438/C/15/Z/Wellcome Trust (Wellcome)
- WT_/Wellcome Trust/United Kingdom
- Studentship/AstraZeneca
- 204845/Z/16/Z/Wellcome Trust (Wellcome)
- postdoctoral research fellowship/Boehringer Ingelheim Fonds (Stiftung für medizinische Grundlagenforschung)
- MR/T032413/1/RCUK | Medical Research Council (MRC)
- BRC/DH | National Institute for Health Research (NIHR)
- 695669/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- Partial Studentship/RCUK | Medical Research Council (MRC)
LinkOut - more resources
Full Text Sources
Miscellaneous

