IL1RAP-specific T cell engager depletes acute myeloid leukemia stem cells
- PMID: 39143574
- PMCID: PMC11325815
- DOI: 10.1186/s13045-024-01586-x
IL1RAP-specific T cell engager depletes acute myeloid leukemia stem cells
Abstract
Background: The interleukin-1 receptor accessory protein (IL1RAP) is highly expressed on acute myeloid leukemia (AML) bulk blasts and leukemic stem cells (LSCs), but not on normal hematopoietic stem cells (HSCs), providing an opportunity to target and eliminate the disease, while sparing normal hematopoiesis. Herein, we report the activity of BIF002, a novel anti-IL1RAP/CD3 T cell engager (TCE) in AML.
Methods: Antibodies to IL1RAP were isolated from CD138+ B cells collected from the immunized mice by optoelectric positioning and single cell sequencing. Individual mouse monoclonal antibodies (mAbs) were produced and characterized, from which we generated BIF002, an anti-human IL1RAP/CD3 TCE using Fab arm exchange. Mutations in human IgG1 Fc were introduced to reduce FcγR binding. The antileukemic activity of BIF002 was characterized in vitro and in vivo using multiple cell lines and patient derived AML samples.
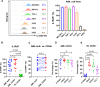
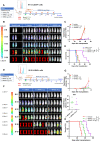
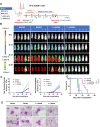
Results: IL1RAP was found to be highly expressed on most human AML cell lines and primary blasts, including CD34+ LSC-enriched subpopulation from patients with both de novo and relapsed/refractory (R/R) leukemia, but not on normal HSCs. In co-culture of T cells from healthy donors and IL1RAPhigh AML cell lines and primary blasts, BIF002 induced dose- and effector-to-target (E:T) ratio-dependent T cell activation and leukemic cell lysis at subnanomolar concentrations. BIF002 administered intravenously along with human T cells led to depletion of leukemic cells, and significantly prolonged survival of IL1RAPhigh MOLM13 or AML patient-derived xenografts with no off-target side effects, compared to controls. Of note, BiF002 effectively redirects T cells to eliminate LSCs, as evidenced by the absence of disease initiation in secondary recipients of bone marrow (BM) from BIF002+T cells-treated donors (median survival not reached; all survived > 200 days) compared with recipients of BM from vehicle- (median survival: 26 days; p = 0.0004) or isotype control antibody+T cells-treated donors (26 days; p = 0.0002).
Conclusions: The novel anti-IL1RAP/CD3 TCE, BIF002, eradicates LSCs and significantly prolongs survival of AML xenografts, representing a promising, novel treatment for AML.
Keywords: Acute myeloid leukemia; IL1RAP; Immunotherapy; Leukemic stem cells; T cell engager.
© 2024. The Author(s).
Conflict of interest statement
Aspects of this work are described and claimed in a pending patent application.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

