Sciatic nerve stimulation alleviates neuropathic pain and associated neuroinflammation in the dorsal root ganglia in a rodent model
- PMID: 39143617
- PMCID: PMC11325705
- DOI: 10.1186/s12967-024-05573-1
Sciatic nerve stimulation alleviates neuropathic pain and associated neuroinflammation in the dorsal root ganglia in a rodent model
Abstract
Background: Satellite glial cells (SGCs) in the dorsal root ganglia (DRG) play a pivotal role in the formation of neuropathic pain (NP). Sciatic nerve stimulation (SNS) neuromodulation was reported to alleviate NP and reduce neuroinflammation. However, the mechanisms underlying SNS in the DRG remain unclear. This study aimed to elucidate the mechanism of electric stimulation in reducing NP, focusing on the DRG.
Methods: L5 nerve root ligation (NRL) NP rat model was studied. Ipsilateral SNS performed 1 day after NRL. Behavioral tests were performed to assess pain phenotypes. NanoString Ncounter technology was used to explore the differentially expressed genes and cellular pathways. Activated SGCs were characterized in vivo and in vitro. The histochemical alterations of SGCs, macrophages, and neurons in DRG were examined in vivo on post-injury day 8.
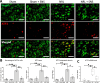
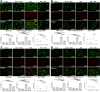
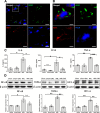
Results: NRL induced NP behaviors including decreased pain threshold and latency on von Frey and Hargreaves tests. We found that following nerve injury, SGCs were hyperactivated, neurotoxic and had increased expression of NP-related ion channels including TRPA1, Cx43, and SGC-neuron gap junctions. Mechanistically, nerve injury induced reciprocal activation of SGCs and M1 macrophages via cytokines including IL-6, CCL3, and TNF-α mediated by the HIF-1α-NF-κB pathways. SNS suppressed SGC hyperactivation, reduced the expression of NP-related ion channels, and induced M2 macrophage polarization, thereby alleviating NP and associated neuroinflammation in the DRG.
Conclusions: NRL induced hyperactivation of SGCs, which had increased expression of NP-related ion channels. Reciprocal activation of SGCs and M1 macrophages surrounding the primary sensory neurons was mediated by the HIF-1α and NF-κB pathways. SNS suppressed SGC hyperactivation and skewed M1 macrophage towards M2. Our findings establish SGC activation as a crucial pathomechanism in the gliopathic alterations in NP, which can be modulated by SNS neuromodulation.
Keywords: Macrophage; Neuropathic pain; Satellite glial cell; Sciatic nerve stimulation.
© 2024. The Author(s).
Conflict of interest statement
W. Liu holds shareholder interest in Aneuvo Biomedical Inc., C-E. Wong and J-S. Lee hold related patents. The other authors declare that they have no competing interests.
Figures





References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

