Interstitial cells of the sip syncytium regulate basal membrane potential in murine gastric corpus
- PMID: 39143726
- PMCID: PMC11587931
- DOI: 10.1096/fj.202400982R
Interstitial cells of the sip syncytium regulate basal membrane potential in murine gastric corpus
Abstract
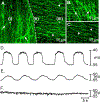
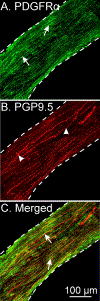
Smooth muscle cells (SMCs), Interstitial cells of Cajal (ICC) and Platelet-derived growth factor receptor α positive (PDGFRα+) cells form an integrated, electrical syncytium within the gastrointestinal (GI) muscular tissues known as the SIP syncytium. Immunohistochemical analysis of gastric corpus muscles showed that c-KIT+/ANO1+ ICC-IM and PDGFRα+ cells were closely apposed to one another in the same anatomical niches. We used intracellular microelectrode recording from corpus muscle bundles to characterize the roles of intramuscular ICC and PDGFRα+ cells in conditioning membrane potentials of gastric muscles. In muscle bundles, that have a relatively higher input impedance than larger muscle strips or sheets, we recorded an ongoing discharge of stochastic fluctuations in membrane potential, previously called unitary potentials or spontaneous transient depolarizations (STDs) and spontaneous transient hyperpolarizations (STHs). We reasoned that STDs should be blocked by antagonists of ANO1, the signature conductance of ICC. Activation of ANO1 has been shown to generate spontaneous transient inward currents (STICs), which are the basis for STDs. Ani9 reduced membrane noise and caused hyperpolarization, but this agent did not block the fluctuations in membrane potential quantitatively. Apamin, an antagonist of small conductance Ca2+-activated K+ channels (SK3), the signature conductance in PDGFRα+ cells, further reduced membrane noise and caused depolarization. Reversing the order of channel antagonists reversed the sequence of depolarization and hyperpolarization. These experiments show that the ongoing discharge of STDs and STHs by ICC and PDGFRα+ cells, respectively, exerts conditioning effects on membrane potentials in the SIP syncytium that would effectively regulate the excitability of SMCs.
Keywords: PDGFRα+ interstitial cells; c‐KIT+ and ANO1+ interstitial cells of Cajal; spontaneous transient depolarizations (STDs); spontaneous transient hyperpolarizations (STHs).
© 2024 Federation of American Societies for Experimental Biology.
Conflict of interest statement
Conflict of Interest Statement
The authors declare no conflicts of interest.
Figures





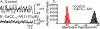



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

