Multiplexed Nanoscopy via Buffer Exchange
- PMID: 39143924
- PMCID: PMC11363122
- DOI: 10.1021/acsnano.4c06829
Multiplexed Nanoscopy via Buffer Exchange
Abstract
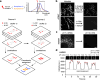
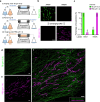
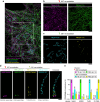
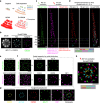
Understanding cellular functions, particularly in their intricate complexity, can greatly benefit from the spatial mapping of diverse molecules through multitarget single-molecule localization microscopy (SMLM). Existing methodologies, primarily restricting the encoding dimensions to color and lifetime or requiring cyclic staining, often involve broad chromatic detection, specialized optical configurations, or sophisticated labeling techniques. Here, we propose a simple approach called buffer-exchange stochastic optical reconstruction microscopy (beSTORM), which introduces an additional dimension to differentiate between single molecules irrespective of their spectral properties. This method leverages the distinguishable photoblinking responses to distinct buffer conditions, offering a straightforward yet effective means of fluorophore discrimination. Through buffer exchanges, beSTORM achieves multitarget SMLM imaging with minimal crosstalk. Direct integration with expansion microscopy (ExM) demonstrates its capability to resolve up to six proteins at the molecular level within a single emission color without chromatic aberration. Overall, beSTORM presents a highly compatible imaging platform, promising significant advancements in highly multiplexed nanoscopy for exploring multiple targets in biological systems with nanoscale precision.
Keywords: (d)STORM; SMLM; expansion microscopy (ExM); multicolor; multiplexed imaging; superresolution microscopy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

