Current and future directions in network biology
- PMID: 39143982
- PMCID: PMC11321866
- DOI: 10.1093/bioadv/vbae099
Current and future directions in network biology
Abstract
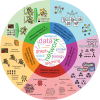
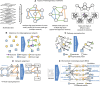
Summary: Network biology is an interdisciplinary field bridging computational and biological sciences that has proved pivotal in advancing the understanding of cellular functions and diseases across biological systems and scales. Although the field has been around for two decades, it remains nascent. It has witnessed rapid evolution, accompanied by emerging challenges. These stem from various factors, notably the growing complexity and volume of data together with the increased diversity of data types describing different tiers of biological organization. We discuss prevailing research directions in network biology, focusing on molecular/cellular networks but also on other biological network types such as biomedical knowledge graphs, patient similarity networks, brain networks, and social/contact networks relevant to disease spread. In more detail, we highlight areas of inference and comparison of biological networks, multimodal data integration and heterogeneous networks, higher-order network analysis, machine learning on networks, and network-based personalized medicine. Following the overview of recent breakthroughs across these five areas, we offer a perspective on future directions of network biology. Additionally, we discuss scientific communities, educational initiatives, and the importance of fostering diversity within the field. This article establishes a roadmap for an immediate and long-term vision for network biology.
Availability and implementation: Not applicable.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
In Section 8, we rely on ISCB’s diversity statistics. These statistics are publicly available, and so there is no conflict of interest. Yet, to remedy any potential perceived conflict of interest, we declare that Predrag Radivojac is the President of ISCB and currently serves on the Board of Directors of ISCB. In addition, Tijana Milenković currently serves on the ISCB Board of Directors and the ISCB EDI Committee. The remaining authors have no conflicts of interest to declare.
Figures




References
-
- Abdar M, Pourpanah F, Hussain S. et al. A review of uncertainty quantification in deep learning: techniques, applications and challenges. Inf Fusion 2021;76:243–97.
-
- Agarwal S, Branson K, Belongie S. Higher order learning with graphs. In: Proceedings of the International Conference on Machine Learning. p. 17–24. New York, NY: Association for Computing Machinery, 2006.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

